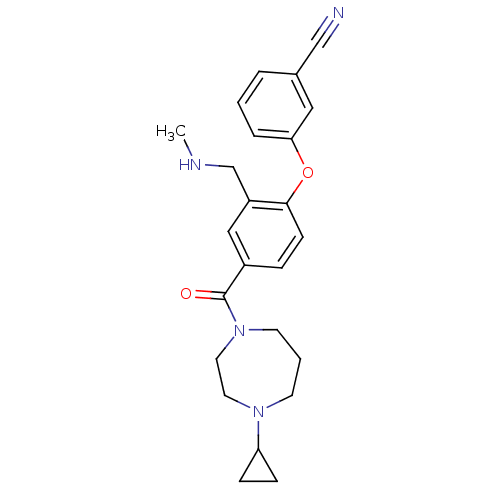
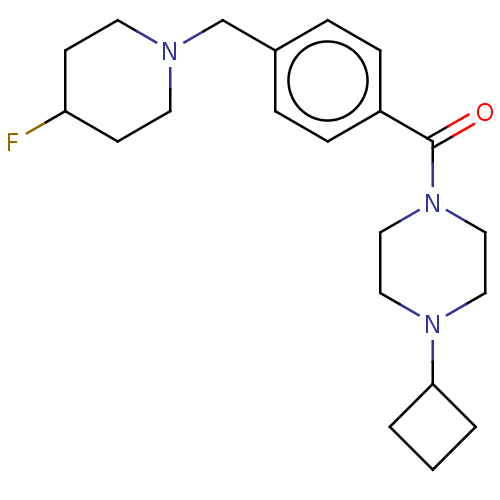
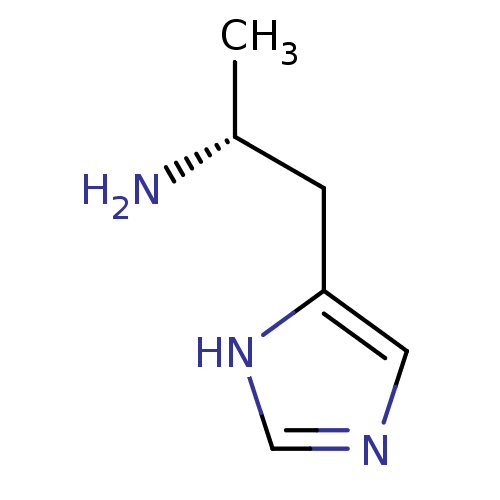
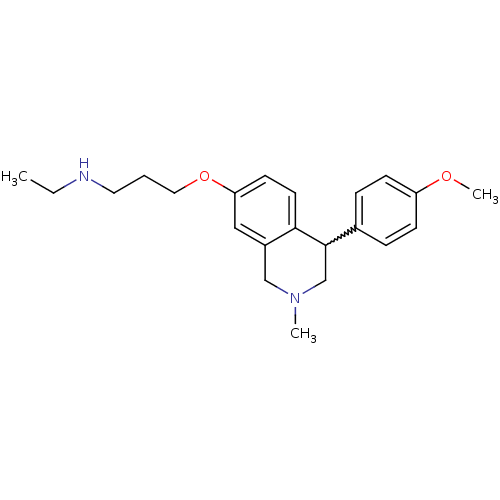
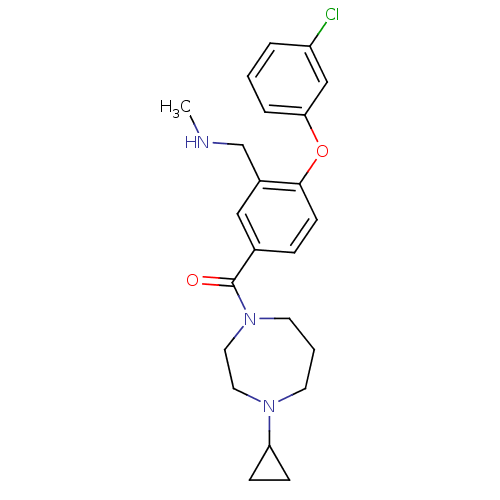
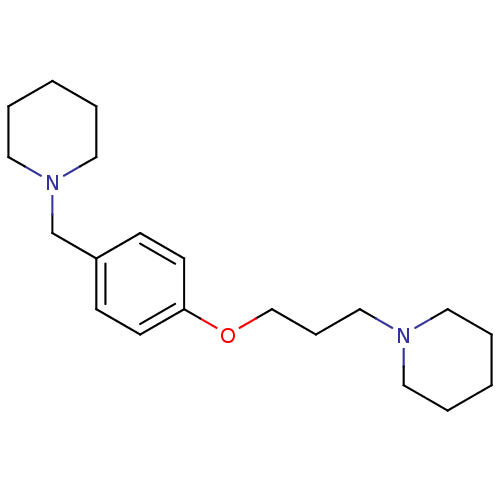
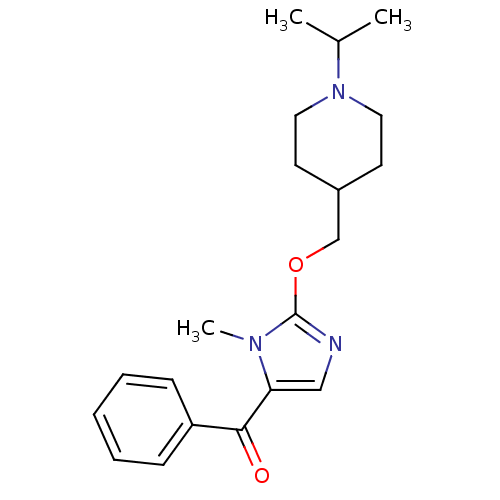
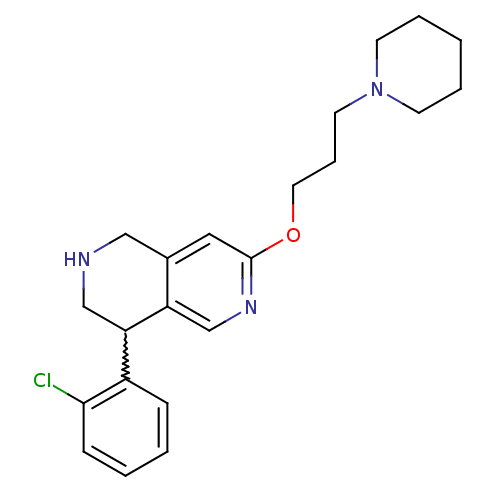
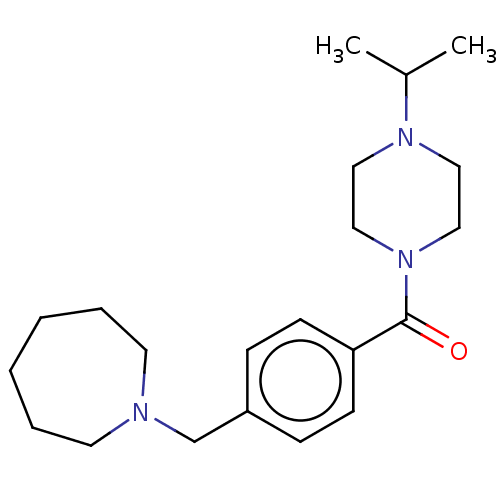
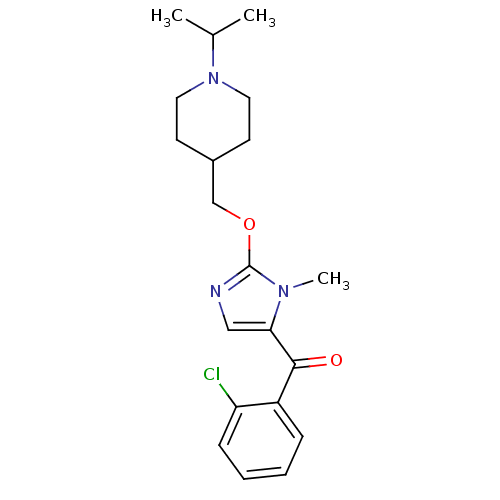
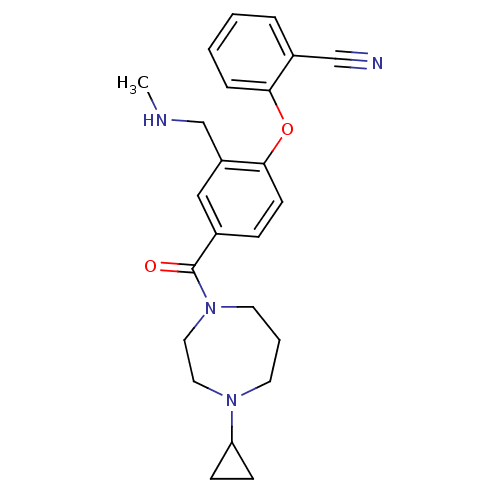
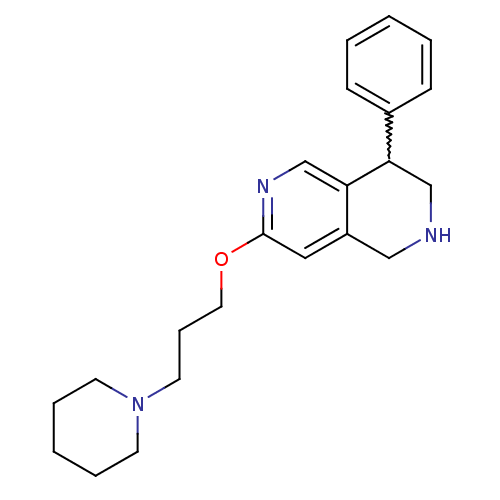
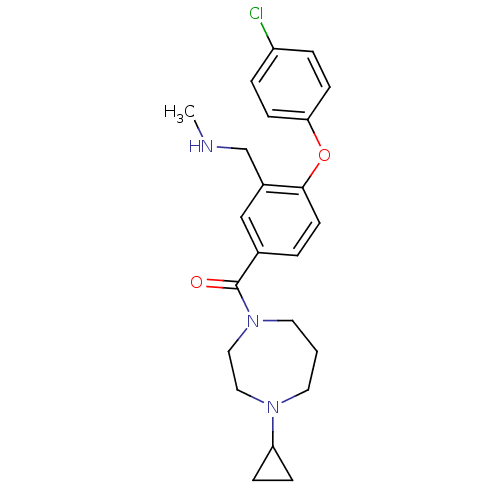
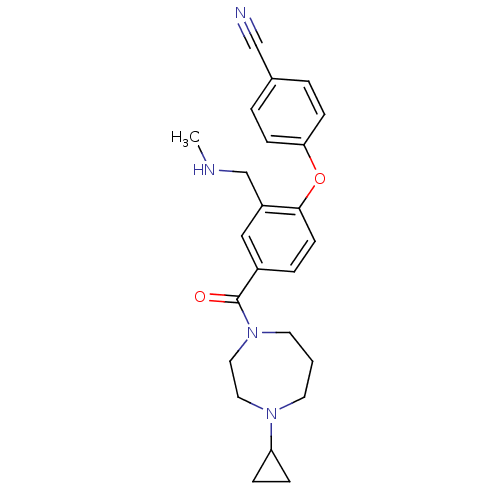
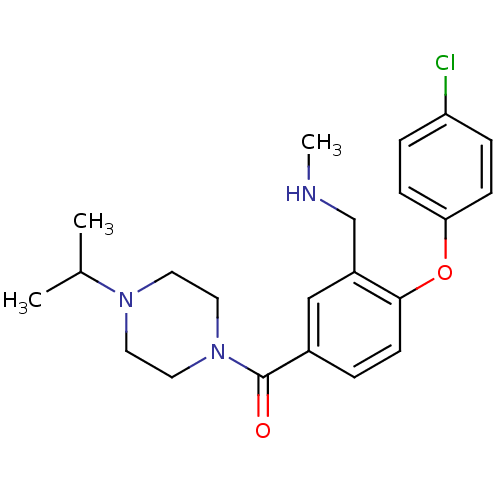
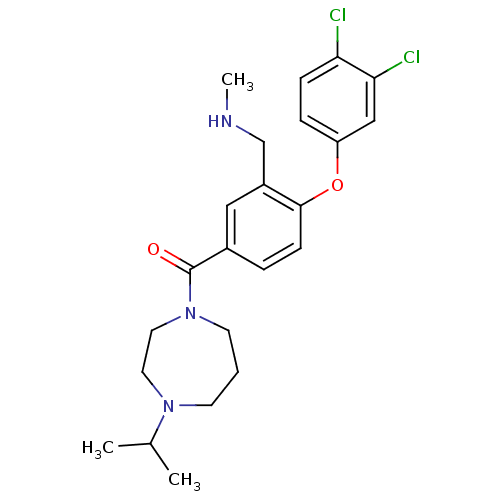
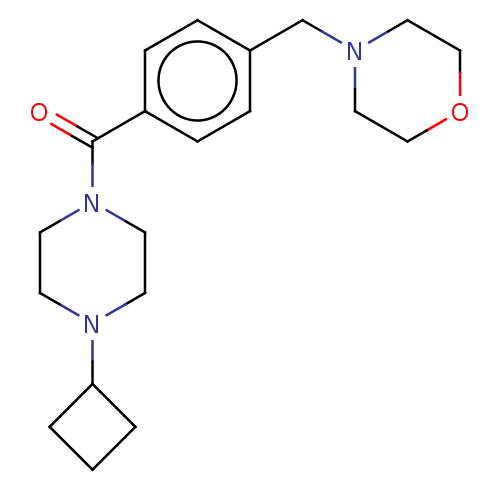
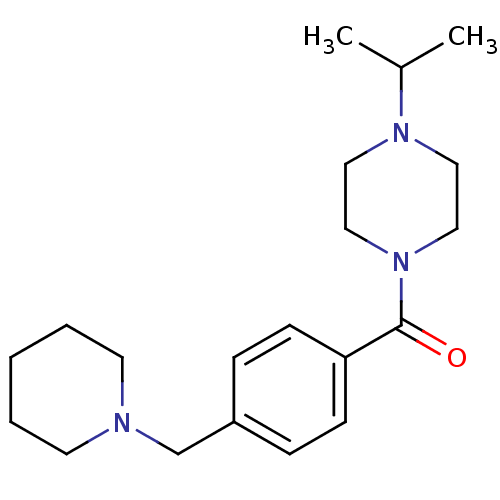
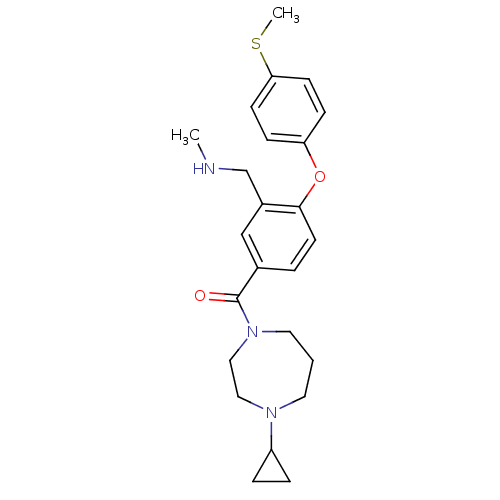
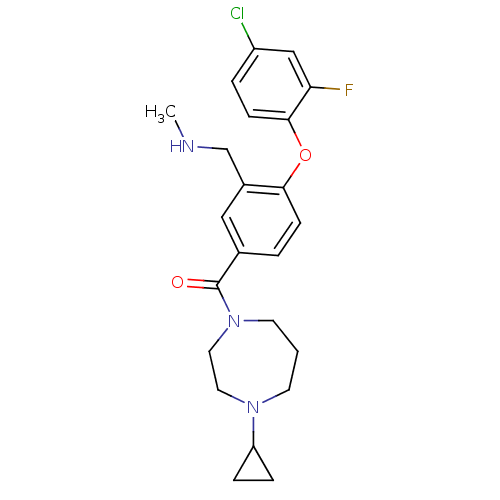
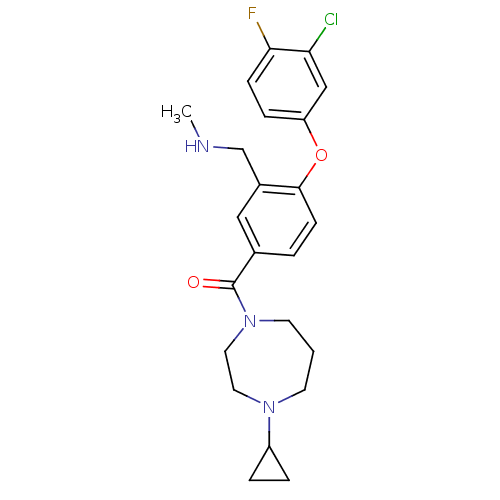
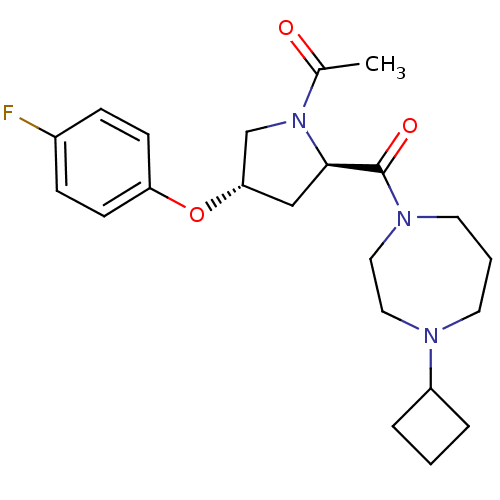
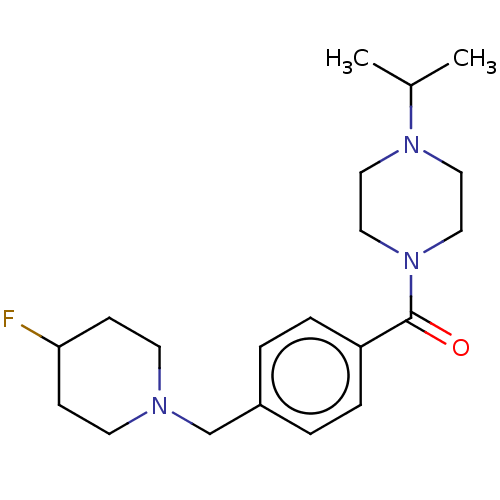
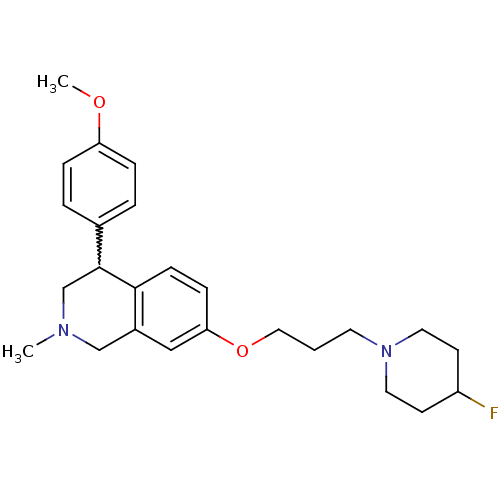
Affinity DataKi: 0.5nMAssay Description:Displacement of (S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-((3H)-1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2 rec...More data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.5nMAssay Description:Inhibition of human histamine H3 receptorMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.700nMAssay Description:Inhibition of human histamine H3 receptorMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.700nMAssay Description:Inhibition of human histamine H3 receptorMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.700nMAssay Description:Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells after 45 minsMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.700nMAssay Description:Binding affinity to the human histamine H3 receptorMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 0.730nMAssay Description:Binding affinity to rat SERTMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.800nMAssay Description:Inhibition of human SERTMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.800nMAssay Description:Inhibition of human histamine H3 receptorMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.800nMAssay Description:Inhibition of human histamine H3 receptorMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.900nMAssay Description:Inhibition of human histamine H3 receptorMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.970nMAssay Description:Displacement of [125I]iodoproxyfan from human recombinant histamine H3 receptor expressed in human SK-N-MC cellsMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.970nMAssay Description:Binding affinity to human histamine H3 receptorMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:Binding affinity to rat SERTMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:Binding affinity to human histamine H3 receptorMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:Inhibition of human histamine H3 receptorMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:Inhibition of human histamine H3 receptorMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:Displacement of N-[3H]alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cellsMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:Inhibition of human histamine H3 receptorMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells after 45 minsMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:Binding affinity to human histamine H3 receptorMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells after 45 minsMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:Displacement of [125I]-iodoproxyfan from human recombinant histamine H3 receptor expressed in human SK-N-MC cells after 1 hr by scintillation countin...More data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:Inhibition of human histamine H3 receptorMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:Inhibition of human histamine H3 receptorMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:Displacement of N-[3H]alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cellsMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1.10nMAssay Description:Inhibition of human histamine H3 receptorMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1.10nMAssay Description:Inhibition of human histamine H3 receptorMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1.10nMAssay Description:Inhibition of human histamine H3 receptorMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1.10nMAssay Description:Inhibition of human histamine H3 receptorMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1.10nMAssay Description:Inhibition of human histamine H3 receptorMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1.20nMAssay Description:Inhibition of human histamine H3 receptorMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1.20nMAssay Description:Binding affinity to human histamine H3 receptorMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1.30nMAssay Description:Binding affinity to human histamine H3 receptorMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 1.30nMAssay Description:Binding affinity to rat SERTMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1.30nMAssay Description:Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells after 45 minsMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1.30nMAssay Description:Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells after 45 minsMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1.40nMAssay Description:Inhibition of human histamine H3 receptorMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 1.40nMAssay Description:Inhibition of rat SERTMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 1.40nMAssay Description:Binding affinity to rat SERTMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1.40nMAssay Description:Displacement of [125I]-iodoproxyfan from human recombinant histamine H3 receptor expressed in human SK-N-MC cells after 1 hr by scintillation countin...More data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1.40nMAssay Description:Inhibition of human histamine H3 receptorMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1.5nMAssay Description:Inhibition of human histamine H3 receptorMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1.5nMAssay Description:Binding affinity to human histamine H3 receptorMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1.5nMAssay Description:Displacement of [125I]-iodoproxyfan from human recombinant histamine H3 receptor expressed in human SK-N-MC cells after 1 hr by scintillation countin...More data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1.5nMAssay Description:Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells after 45 minsMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 1.5nMAssay Description:Binding affinity to rat SERTMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1.60nMAssay Description:Binding affinity to human histamine H3 receptorMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 1.60nMAssay Description:Binding affinity to rat SERTMore data for this Ligand-Target Pair