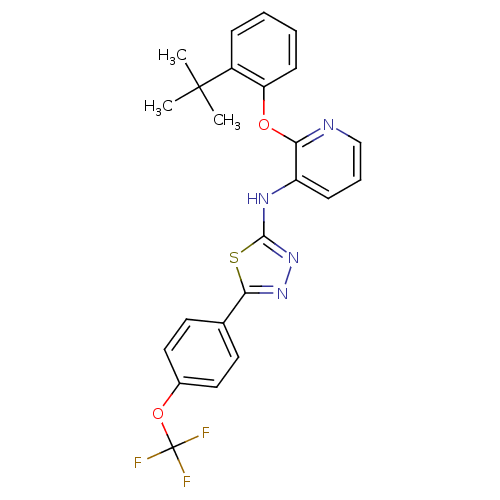
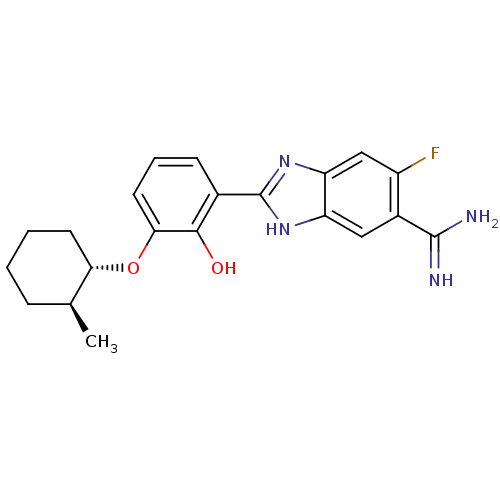
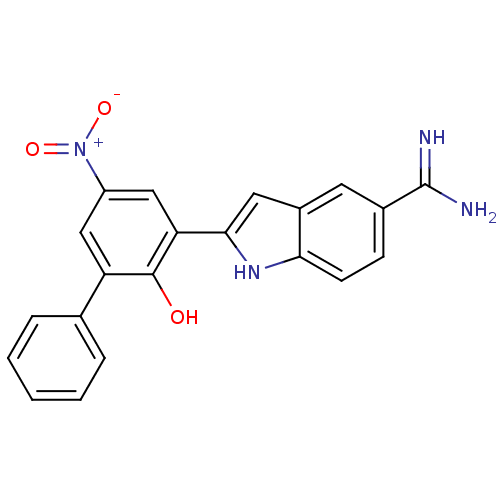
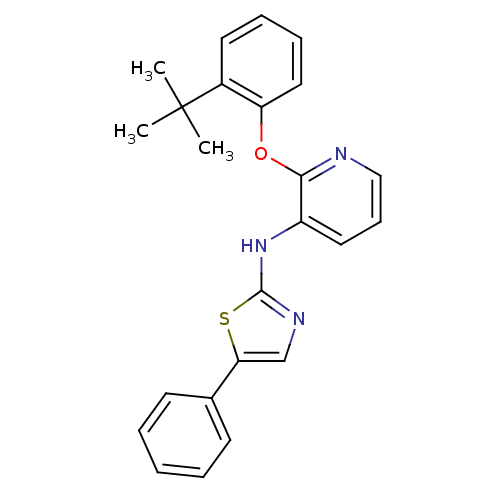
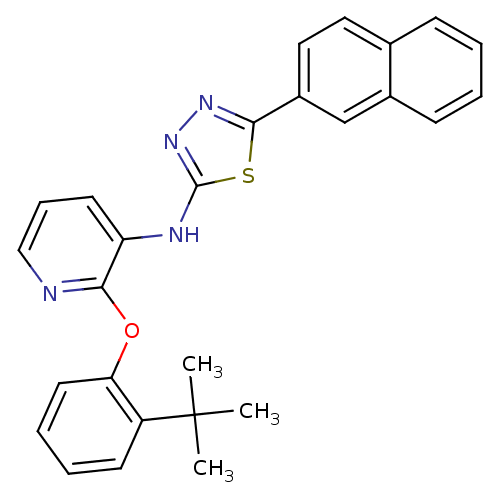
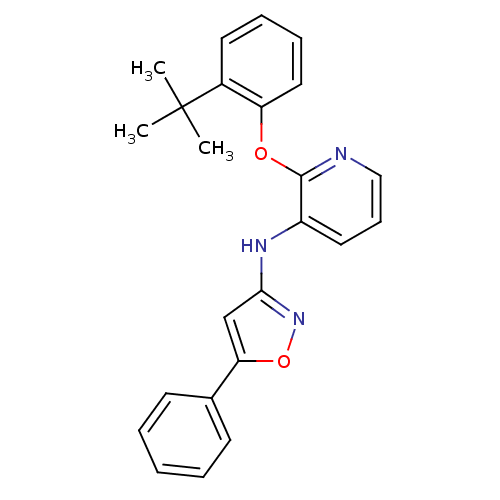
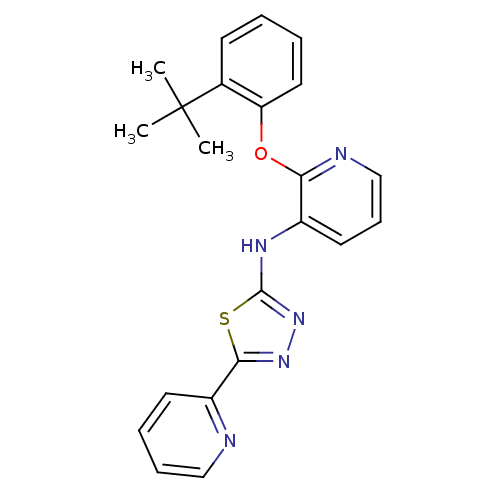
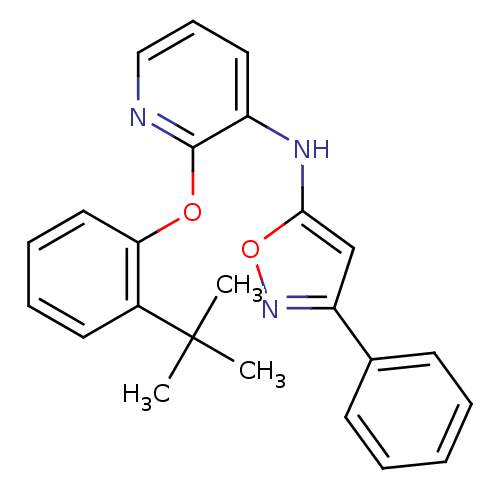
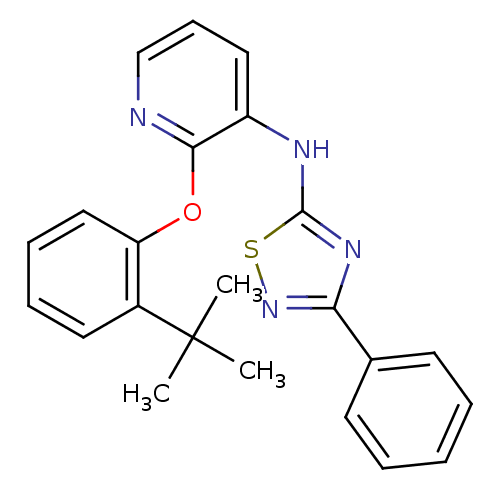
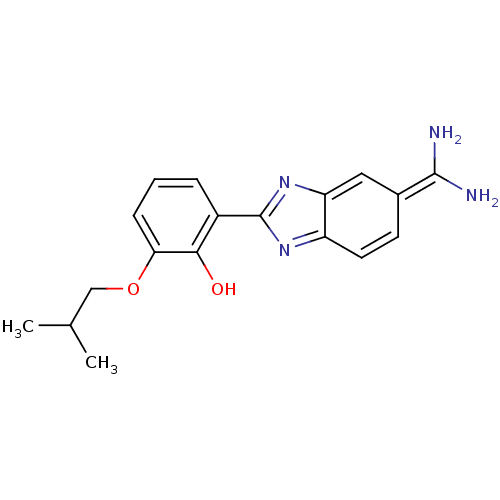
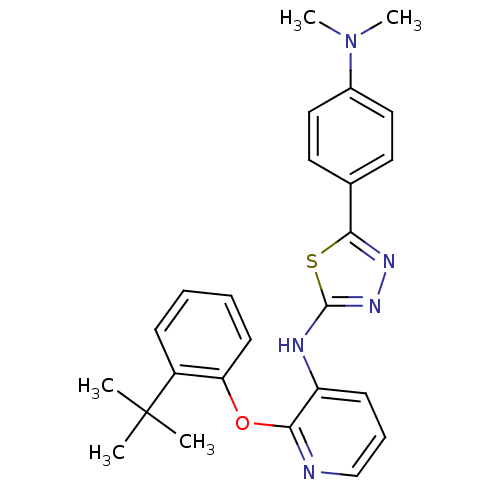
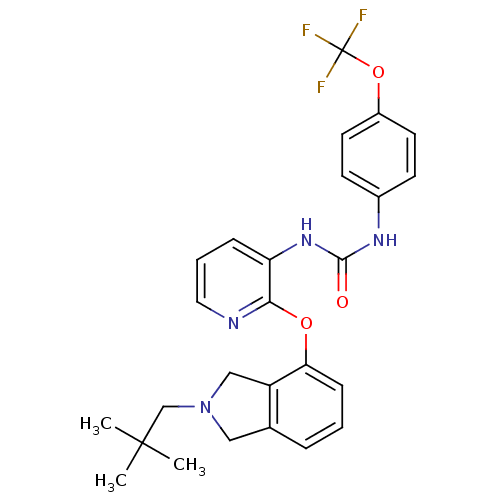
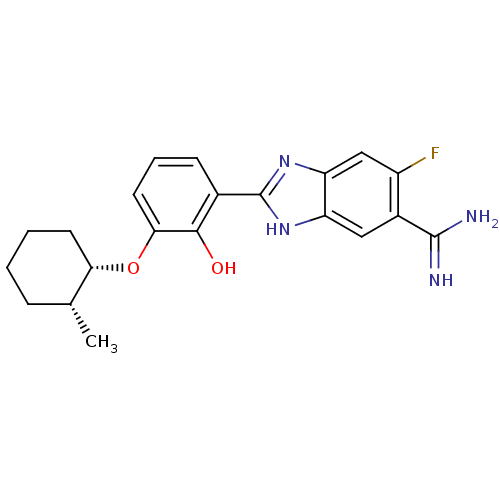
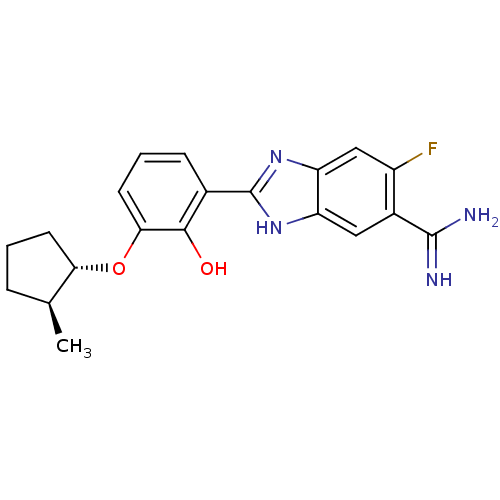
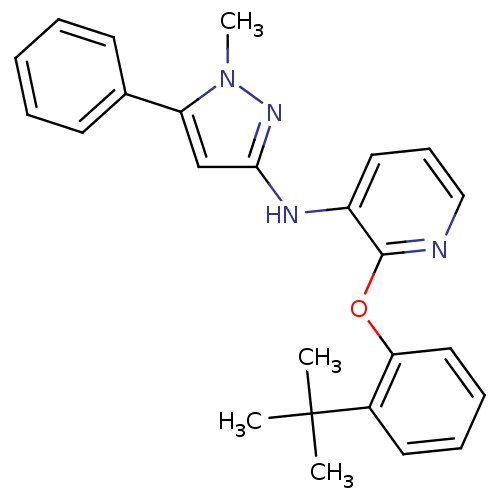
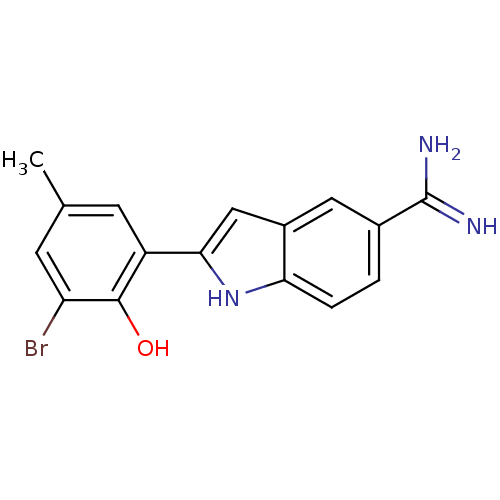
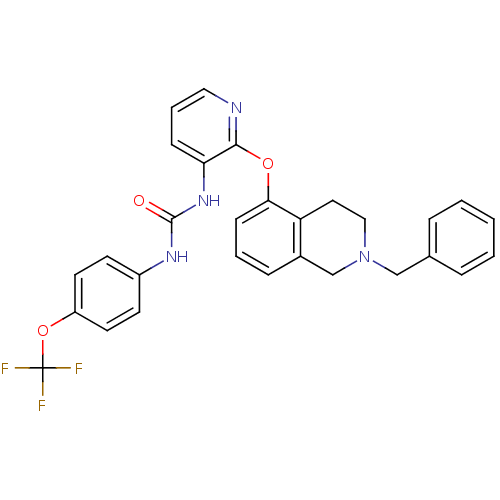
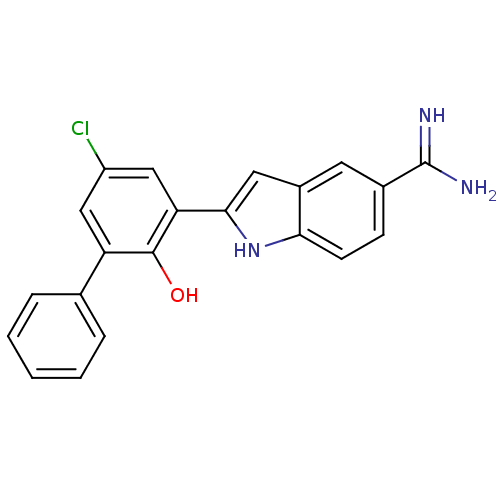
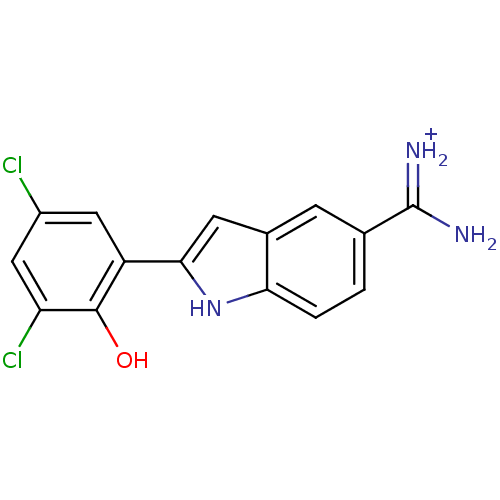
Affinity DataKi: 7nMAssay Description:Displacement of [beta-33P]-2MeS-ADP from human P2Y1 receptor expressed in HEK293 cells after 1 hr by scintillation counting analysisMore data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
Axys Pharmaceuticals
Curated by ChEMBL
Axys Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 8nMAssay Description:Inhibitory concentration against Human Serine Protease Urokinase Plasminogen ActivatorMore data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
Axys Pharmaceuticals
Curated by ChEMBL
Axys Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 8nM ΔG°: -45.7kJ/molepH: 7.4 T: 2°CAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
Axys Pharmaceuticals
Curated by ChEMBL
Axys Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 8nMAssay Description:Inhibition of urokinase-type plasminogen activatorMore data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
Axys Pharmaceuticals
Curated by ChEMBL
Axys Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 9nM ΔG°: -45.5kJ/molepH: 7.4 T: 2°CAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:Displacement of [beta-33P]-2MeS-ADP from human P2Y1 receptor expressed in HEK293 cells after 1 hr by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataKi: 11nMAssay Description:Displacement of [beta-33P]-2MeS-ADP from human P2Y1 receptor transfected in HEK293 cells after 1 hr by scintillation counting methodMore data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
Axys Pharmaceuticals
Curated by ChEMBL
Axys Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 11nMAssay Description:Inhibition of urokinase-type plasminogen activatorMore data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
Axys Pharmaceuticals
Curated by ChEMBL
Axys Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 11nM ΔG°: -45.0kJ/molepH: 7.4 T: 2°CAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
Affinity DataKi: 11nMAssay Description:Displacement of [beta-33P]-2MeS-ADP from human P2Y1 receptor expressed in HEK293 cells after 1 hr by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataKi: 14nMAssay Description:Binding affinity against human coagulation factor XMore data for this Ligand-Target Pair
Affinity DataKi: 15nMAssay Description:Displacement of [beta-33P]-2MeS-ADP from human P2Y1 receptor expressed in HEK293 cells after 1 hr by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataKi: 15nMAssay Description:Displacement of [beta-33P]-2MeS-ADP from human P2Y1 receptor expressed in HEK293 cells after 1 hr by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataKi: 16nMAssay Description:Displacement of [beta-33P]-2MeS-ADP from human P2Y1 receptor transfected in HEK293 cells after 1 hr by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 18nMAssay Description:Displacement of [beta-33P]-2MeS-ADP from human P2Y1 receptor expressed in HEK293 cells after 1 hr by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataKi: 19nMAssay Description:Inhibition of Human Serine Protease tissue type Plasminogen Activator (t-PA).More data for this Ligand-Target Pair
Affinity DataKi: 19nMAssay Description:Displacement of [beta-33P]-2MeS-ADP from human P2Y1 receptor expressed in HEK293 cells after 1 hr by scintillation counting analysisMore data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
Axys Pharmaceuticals
Curated by ChEMBL
Axys Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 20nM ΔG°: -43.5kJ/molepH: 7.4 T: 2°CAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
Affinity DataKi: 21nMAssay Description:Displacement of [beta-33P]-2MeS-ADP from human P2Y1 receptor expressed in HEK293 cells after 1 hr by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataKi: 23nMAssay Description:Displacement of [beta-33P]-2MeS-ADP from human P2Y1 receptor expressed in HEK293 cells after 1 hr by scintillation counting analysisMore data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
Axys Pharmaceuticals
Curated by ChEMBL
Axys Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 25nM ΔG°: -43.0kJ/molepH: 8.2 T: 2°CAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
Axys Pharmaceuticals
Curated by ChEMBL
Axys Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 25nMAssay Description:ComInhibition of Human Serine Protease Urokinase Plasminogen Activator (u-PA).More data for this Ligand-Target Pair
Affinity DataKi: 25nMAssay Description:Displacement of [beta-33P]-2MeS-ADP from human P2Y1 receptor expressed in HEK293 cells after 1 hr by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataKi: 26nMAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
Affinity DataKi: 27nMAssay Description:Displacement of [beta-33P]-2MeS-ADP from human P2Y1 receptor expressed in HEK293 cells after 1 hr by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataKi: 29nMAssay Description:Displacement of [beta-33P]-2MeS-ADP from human P2Y1 receptor transfected in HEK293 cells after 1 hr by scintillation counting methodMore data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
Axys Pharmaceuticals
Curated by ChEMBL
Axys Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 32nMAssay Description:Inhibition of urokinase-type plasminogen activatorMore data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
Axys Pharmaceuticals
Curated by ChEMBL
Axys Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 32nMAssay Description:Inhibition of urokinase-type plasminogen activatorMore data for this Ligand-Target Pair
Affinity DataKi: 35nMAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
Affinity DataKi: 35nMAssay Description:Inihibtion of Human Serine Protease tissue type Plasminogen ActivatorMore data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
Axys Pharmaceuticals
Curated by ChEMBL
Axys Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 35nMAssay Description:Inhibitory concentration against Human Serine Protease Urokinase Plasminogen ActivatorMore data for this Ligand-Target Pair
Affinity DataKi: 35nMAssay Description:Inhibition of tissue-type plasminogen activatorMore data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
Axys Pharmaceuticals
Curated by ChEMBL
Axys Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 38nMAssay Description:Inhibitory concentration against Human Serine Protease Urokinase Plasminogen ActivatorMore data for this Ligand-Target Pair
Affinity DataKi: 41nMAssay Description:Displacement of [beta-33P]-2MeS-ADP from human P2Y1 receptor expressed in HEK293 cells after 1 hr by scintillation counting analysisMore data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
Axys Pharmaceuticals
Curated by ChEMBL
Axys Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 43nMAssay Description:Inhibitory concentration against Human Serine Protease Urokinase Plasminogen ActivatorMore data for this Ligand-Target Pair
Affinity DataKi: 43nMAssay Description:Binding affinity against human coagulation factor XMore data for this Ligand-Target Pair
Affinity DataKi: 45nMAssay Description:Displacement of [beta-33P]-2MeS-ADP from human P2Y1 receptor expressed in HEK293 cells after 1 hr by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataKi: 52nMAssay Description:Binding affinity against human coagulation factor XMore data for this Ligand-Target Pair
Affinity DataKi: 55nMAssay Description:Displacement of [beta-33P]-2MeS-ADP from human P2Y1 receptor transfected in HEK293 cells after 1 hr by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 55nMAssay Description:Displacement of [beta-33P]-2MeS-ADP from human P2Y1 receptor expressed in HEK293 cells after 1 hr by scintillation counting analysisMore data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
Axys Pharmaceuticals
Curated by ChEMBL
Axys Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 55nMAssay Description:Inhibitory concentration against Human Serine Protease Urokinase Plasminogen ActivatorMore data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
Axys Pharmaceuticals
Curated by ChEMBL
Axys Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 55nMAssay Description:ComInhibition of Human Serine Protease Urokinase Plasminogen Activator (u-PA).More data for this Ligand-Target Pair
Affinity DataKi: 57nMAssay Description:Displacement of [beta-33P]-2MeS-ADP from human P2Y1 receptor transfected in HEK293 cells after 1 hr by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 59nMAssay Description:Displacement of [beta-33P]-2MeS-ADP from human P2Y1 receptor expressed in HEK293 cells after 1 hr by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataKi: 60nMAssay Description:Displacement of [beta-33P]-2MeS-ADP from human P2Y1 receptor transfected in HEK293 cells after 1 hr by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 68nMAssay Description:Displacement of [beta-33P]-2MeS-ADP from human P2Y1 receptor transfected in HEK293 cells after 1 hr by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 70nMAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
Affinity DataKi: 70nMAssay Description:Displacement of [beta-33P]-2MeS-ADP from human P2Y1 receptor expressed in HEK293 cells after 1 hr by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataKi: 75nMAssay Description:Inhibition of Human Serine Protease Trypsin.More data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
Axys Pharmaceuticals
Curated by ChEMBL
Axys Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 77nM ΔG°: -40.2kJ/molepH: 7.4 T: 2°CAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair


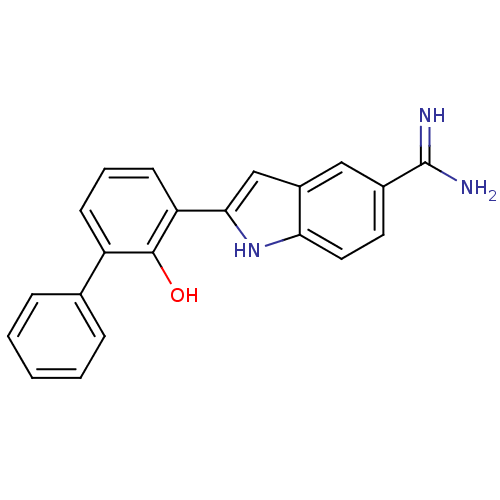
 3D Structure (crystal)
3D Structure (crystal)