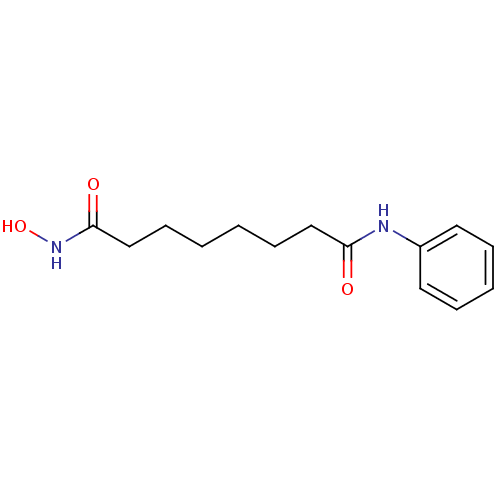
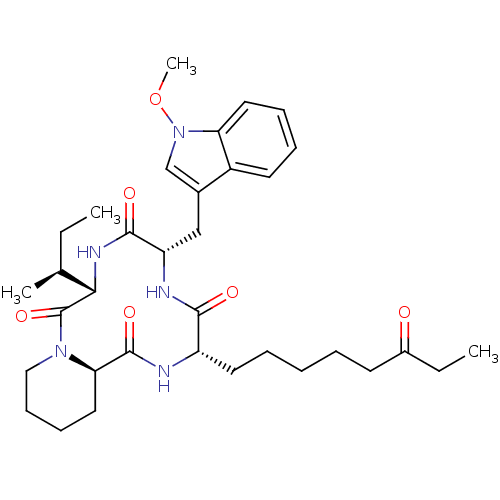
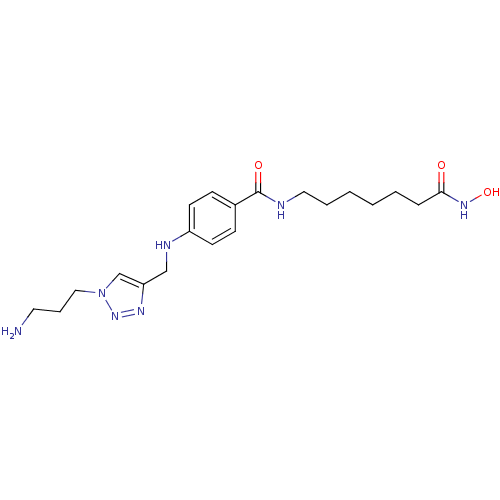
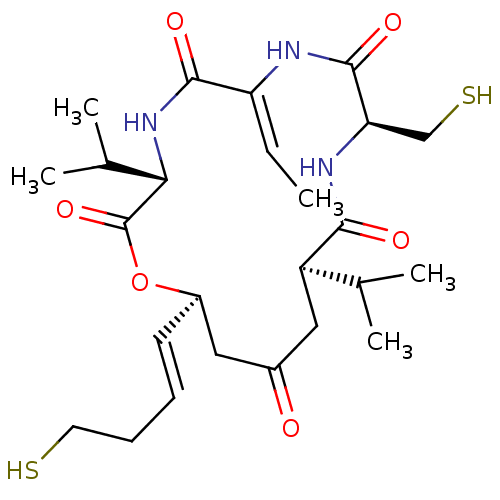
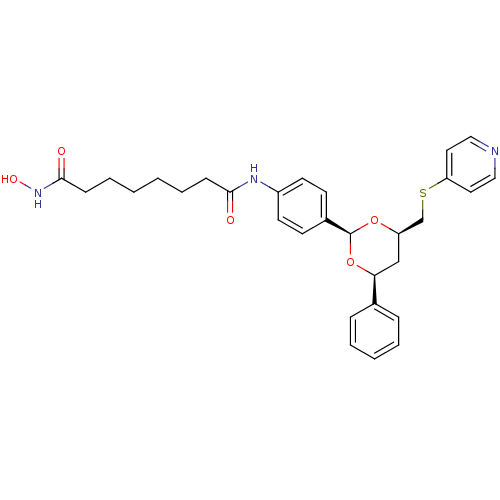
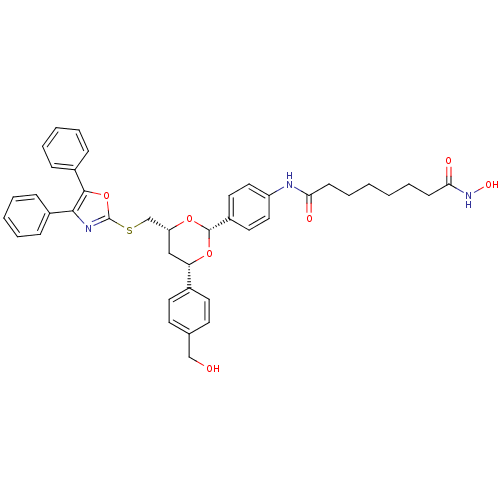
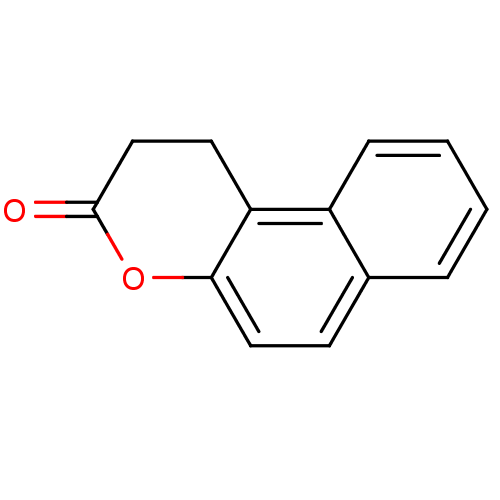
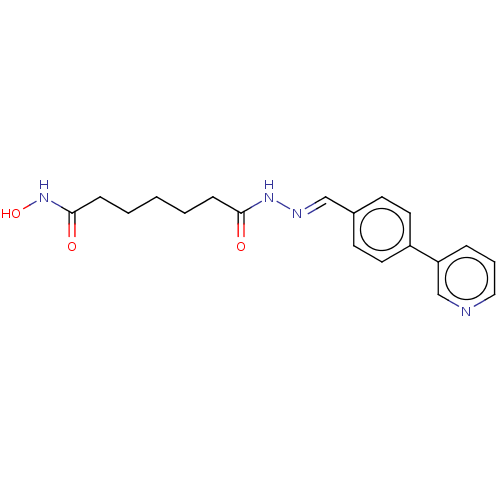
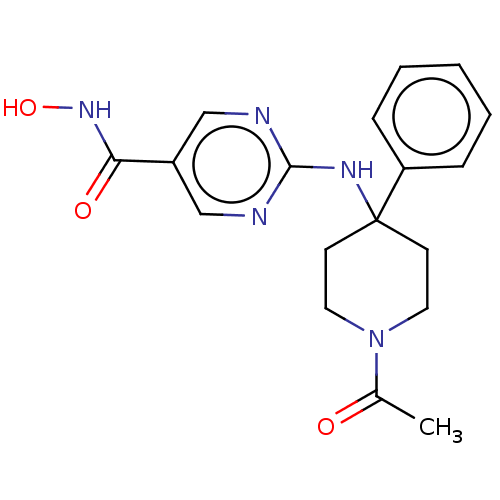
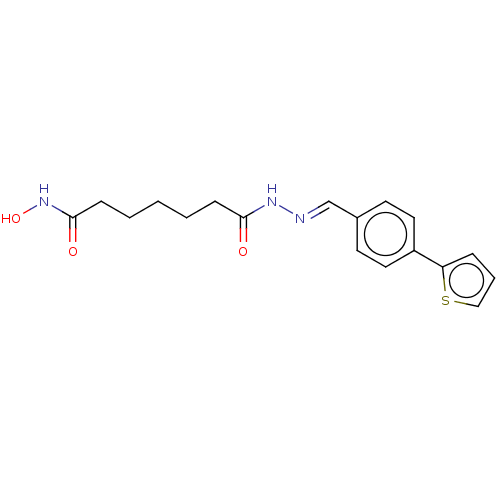
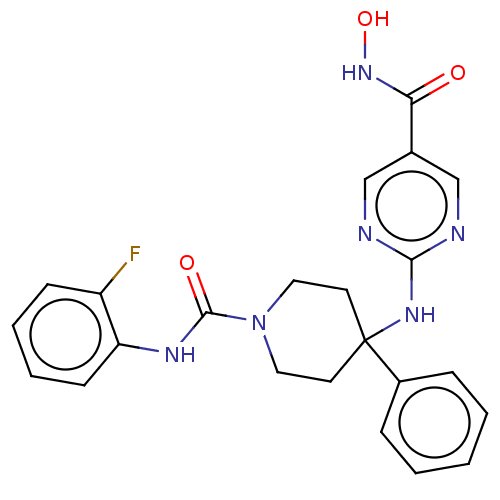
TargetHistone deacetylase 6(Homo sapiens (Human))
The Broad Institute Of Harvard And Mit
Curated by ChEMBL
The Broad Institute Of Harvard And Mit
Curated by ChEMBL
Affinity DataKi: 0.130nMAssay Description:Displacement of fluorescent 5-(3-(3-(4-((4-((7-(hydroxyamino)-7-oxoheptyl)carbamoyl)phenylamino)methyl)-1H-1,2,3-triazol-1-yl)propyl)thioureido)-2-(3...More data for this Ligand-Target Pair
TargetHistone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2)(Homo sapiens (Human))
The Broad Institute Of Harvard And Mit
Curated by ChEMBL
The Broad Institute Of Harvard And Mit
Curated by ChEMBL
Affinity DataKi: 0.260nMAssay Description:Displacement of fluorescent 5-(3-(3-(4-((4-((7-(hydroxyamino)-7-oxoheptyl)carbamoyl)phenylamino)methyl)-1H-1,2,3-triazol-1-yl)propyl)thioureido)-2-(3...More data for this Ligand-Target Pair
TargetHistone deacetylase 6(Homo sapiens (Human))
The Broad Institute Of Harvard And Mit
Curated by ChEMBL
The Broad Institute Of Harvard And Mit
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:Displacement of fluorescent 5-(3-(3-(4-((4-((7-(hydroxyamino)-7-oxoheptyl)carbamoyl)phenylamino)methyl)-1H-1,2,3-triazol-1-yl)propyl)thioureido)-2-(3...More data for this Ligand-Target Pair
TargetHistone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2)(Homo sapiens (Human))
The Broad Institute Of Harvard And Mit
Curated by ChEMBL
The Broad Institute Of Harvard And Mit
Curated by ChEMBL
Affinity DataKi: 1.5nMAssay Description:Displacement of fluorescent 5-(3-(3-(4-((4-((7-(hydroxyamino)-7-oxoheptyl)carbamoyl)phenylamino)methyl)-1H-1,2,3-triazol-1-yl)propyl)thioureido)-2-(3...More data for this Ligand-Target Pair
TargetHistone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2)(Homo sapiens (Human))
The Broad Institute Of Harvard And Mit
Curated by ChEMBL
The Broad Institute Of Harvard And Mit
Curated by ChEMBL
Affinity DataKi: 2nMAssay Description:Displacement of fluorescent 5-(3-(3-(4-((4-((7-(hydroxyamino)-7-oxoheptyl)carbamoyl)phenylamino)methyl)-1H-1,2,3-triazol-1-yl)propyl)thioureido)-2-(3...More data for this Ligand-Target Pair
TargetHistone deacetylase 6(Homo sapiens (Human))
The Broad Institute Of Harvard And Mit
Curated by ChEMBL
The Broad Institute Of Harvard And Mit
Curated by ChEMBL
Affinity DataKi: 6.60nMAssay Description:Displacement of fluorescent 5-(3-(3-(4-((4-((7-(hydroxyamino)-7-oxoheptyl)carbamoyl)phenylamino)methyl)-1H-1,2,3-triazol-1-yl)propyl)thioureido)-2-(3...More data for this Ligand-Target Pair
TargetHistone deacetylase 6(Homo sapiens (Human))
The Broad Institute Of Harvard And Mit
Curated by ChEMBL
The Broad Institute Of Harvard And Mit
Curated by ChEMBL
Affinity DataKi: 21nM ΔG°: -43.4kJ/molepH: 7.4 T: 2°CAssay Description:To assess the effect of test compounds on histone deacetylase enzyme function in Vitro, a fluorometric assay was performed using HDAC, which incubate...More data for this Ligand-Target Pair
TargetHistone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2)(Homo sapiens (Human))
The Broad Institute Of Harvard And Mit
Curated by ChEMBL
The Broad Institute Of Harvard And Mit
Curated by ChEMBL
Affinity DataKi: 23.1nMAssay Description:Displacement of fluorescent 5-(3-(3-(4-((4-((7-(hydroxyamino)-7-oxoheptyl)carbamoyl)phenylamino)methyl)-1H-1,2,3-triazol-1-yl)propyl)thioureido)-2-(3...More data for this Ligand-Target Pair
TargetHistone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2)(Homo sapiens (Human))
The Broad Institute Of Harvard And Mit
Curated by ChEMBL
The Broad Institute Of Harvard And Mit
Curated by ChEMBL
Affinity DataKi: 24.9nMAssay Description:Displacement of fluorescent 5-(3-(3-(4-((4-((7-(hydroxyamino)-7-oxoheptyl)carbamoyl)phenylamino)methyl)-1H-1,2,3-triazol-1-yl)propyl)thioureido)-2-(3...More data for this Ligand-Target Pair
TargetHistone deacetylase 6(Homo sapiens (Human))
The Broad Institute Of Harvard And Mit
Curated by ChEMBL
The Broad Institute Of Harvard And Mit
Curated by ChEMBL
Affinity DataKi: 28nM ΔG°: -42.7kJ/molepH: 7.4 T: 2°CAssay Description:To assess the effect of test compounds on histone deacetylase enzyme function in Vitro, a fluorometric assay was performed using HDAC, which incubate...More data for this Ligand-Target Pair
Affinity DataKi: 48nM ΔG°: -41.4kJ/molepH: 7.4 T: 2°CAssay Description:To assess the effect of test compounds on histone deacetylase enzyme function in Vitro, a fluorometric assay was performed using HDAC, which incubate...More data for this Ligand-Target Pair
Affinity DataKi: 88nM ΔG°: -39.9kJ/molepH: 7.4 T: 2°CAssay Description:To assess the effect of test compounds on histone deacetylase enzyme function in Vitro, a fluorometric assay was performed using HDAC, which incubate...More data for this Ligand-Target Pair
TargetHistone deacetylase 6(Homo sapiens (Human))
The Broad Institute Of Harvard And Mit
Curated by ChEMBL
The Broad Institute Of Harvard And Mit
Curated by ChEMBL
Affinity DataKi: 123nMAssay Description:Displacement of fluorescent 5-(3-(3-(4-((4-((7-(hydroxyamino)-7-oxoheptyl)carbamoyl)phenylamino)methyl)-1H-1,2,3-triazol-1-yl)propyl)thioureido)-2-(3...More data for this Ligand-Target Pair
TargetHistone deacetylase 6(Homo sapiens (Human))
The Broad Institute Of Harvard And Mit
Curated by ChEMBL
The Broad Institute Of Harvard And Mit
Curated by ChEMBL
Affinity DataKi: 142nM ΔG°: -38.7kJ/molepH: 7.4 T: 2°CAssay Description:To assess the effect of test compounds on histone deacetylase enzyme function in Vitro, a fluorometric assay was performed using HDAC, which incubate...More data for this Ligand-Target Pair
TargetHistone deacetylase 6(Homo sapiens (Human))
The Broad Institute Of Harvard And Mit
Curated by ChEMBL
The Broad Institute Of Harvard And Mit
Curated by ChEMBL
Affinity DataKi: 300nMAssay Description:Displacement of fluorescent 5-(3-(3-(4-((4-((7-(hydroxyamino)-7-oxoheptyl)carbamoyl)phenylamino)methyl)-1H-1,2,3-triazol-1-yl)propyl)thioureido)-2-(3...More data for this Ligand-Target Pair
Affinity DataKi: 995nM ΔG°: -33.9kJ/molepH: 7.4 T: 2°CAssay Description:To assess the effect of test compounds on histone deacetylase enzyme function in Vitro, a fluorometric assay was performed using HDAC, which incubate...More data for this Ligand-Target Pair
Affinity DataKi: 2.00E+3nM ΔG°: -32.2kJ/molepH: 7.4 T: 2°CAssay Description:To assess the effect of test compounds on histone deacetylase enzyme function in Vitro, a fluorometric assay was performed using HDAC, which incubate...More data for this Ligand-Target Pair
Affinity DataKi: 6.10E+3nM ΔG°: -29.5kJ/molepH: 7.4 T: 2°CAssay Description:To assess the effect of test compounds on histone deacetylase enzyme function in Vitro, a fluorometric assay was performed using HDAC, which incubate...More data for this Ligand-Target Pair
Affinity DataKi: 6.30E+3nM ΔG°: -29.4kJ/molepH: 7.4 T: 2°CAssay Description:To assess the effect of test compounds on histone deacetylase enzyme function in Vitro, a fluorometric assay was performed using HDAC, which incubate...More data for this Ligand-Target Pair
TargetHistone deacetylase 6(Homo sapiens (Human))
The Broad Institute Of Harvard And Mit
Curated by ChEMBL
The Broad Institute Of Harvard And Mit
Curated by ChEMBL
Affinity DataKi: >7.50E+4nMAssay Description:Displacement of fluorescent 5-(3-(3-(4-((4-((7-(hydroxyamino)-7-oxoheptyl)carbamoyl)phenylamino)methyl)-1H-1,2,3-triazol-1-yl)propyl)thioureido)-2-(3...More data for this Ligand-Target Pair
TargetHistone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2)(Homo sapiens (Human))
The Broad Institute Of Harvard And Mit
Curated by ChEMBL
The Broad Institute Of Harvard And Mit
Curated by ChEMBL
Affinity DataKi: >7.50E+4nMAssay Description:Displacement of fluorescent 5-(3-(3-(4-((4-((7-(hydroxyamino)-7-oxoheptyl)carbamoyl)phenylamino)methyl)-1H-1,2,3-triazol-1-yl)propyl)thioureido)-2-(3...More data for this Ligand-Target Pair
Affinity DataIC50: 0.150nMAssay Description:Inhibition of HDAC1More data for this Ligand-Target Pair
TargetHistone deacetylase 6(Homo sapiens (Human))
The Broad Institute Of Harvard And Mit
Curated by ChEMBL
The Broad Institute Of Harvard And Mit
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:Inhibition of human recombinant HDAC6 using acetyllysine tripeptide coupled with 7-amino-4-methylcoumarin as substrate by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:Inhibition of HDAC3More data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:Inhibition of human recombinant HDAC1 using acetyllysine tripeptide coupled with 7-amino-4-methylcoumarin as substrate by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:Inhibition of HDAC3More data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMpH: 7.5 T: 2°CAssay Description:Recombinant pfHDAC-1 was assayed with substrate in the presence of test compound. The substrate concentration was kept constant at 125 uM while the c...More data for this Ligand-Target Pair
TargetHistone deacetylase 6(Homo sapiens (Human))
The Broad Institute Of Harvard And Mit
Curated by ChEMBL
The Broad Institute Of Harvard And Mit
Curated by ChEMBL
Affinity DataIC50: 0.600nMAssay Description:Inhibition of human recombinant HDAC6 using acetyllysine tripeptide coupled with 7-amino-4-methylcoumarin as substrate by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMAssay Description:Inhibition of human recombinant HDAC1 using acetyllysine tripeptide coupled with 7-amino-4-methylcoumarin as substrate by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMAssay Description:Inhibition of HDAC1More data for this Ligand-Target Pair
Affinity DataIC50: 0.800nMAssay Description:Inhibition of human recombinant HDAC3 using acetyllysine tripeptide coupled with 7-amino-4-methylcoumarin as substrate by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:Compounds for testing were diluted in DMSO to 50 fold the final concentration and a ten point three fold dilution series was made. The compounds were...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human recombinant HDAC2 using acetyllysine tripeptide coupled with 7-amino-4-methylcoumarin as substrate by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMpH: 7.5 T: 2°CAssay Description:Recombinant pfHDAC-1 was assayed with substrate in the presence of test compound. The substrate concentration was kept constant at 125 uM while the c...More data for this Ligand-Target Pair
TargetHistone deacetylase 6(Homo sapiens (Human))
The Broad Institute Of Harvard And Mit
Curated by ChEMBL
The Broad Institute Of Harvard And Mit
Curated by ChEMBL
Affinity DataIC50: 1nMpH: 7.4 T: 2°CAssay Description:Compounds for testing were diluted in DMSO to 50 fold the final concentration and a ten point three fold dilution series was made. The compounds were...More data for this Ligand-Target Pair
TargetHistone deacetylase 6(Homo sapiens (Human))
The Broad Institute Of Harvard And Mit
Curated by ChEMBL
The Broad Institute Of Harvard And Mit
Curated by ChEMBL
Affinity DataIC50: 1nMpH: 7.4 T: 2°CAssay Description:Compounds for testing were diluted in DMSO to 50 fold the final concentration and a ten point three fold dilution series was made. The compounds were...More data for this Ligand-Target Pair
TargetHistone deacetylase 6(Homo sapiens (Human))
The Broad Institute Of Harvard And Mit
Curated by ChEMBL
The Broad Institute Of Harvard And Mit
Curated by ChEMBL
Affinity DataIC50: 1nMpH: 7.4 T: 2°CAssay Description:Compounds for testing were diluted in DMSO to 50 fold the final concentration and a ten point three fold dilution series was made. The compounds were...More data for this Ligand-Target Pair
TargetHistone deacetylase 6(Homo sapiens (Human))
The Broad Institute Of Harvard And Mit
Curated by ChEMBL
The Broad Institute Of Harvard And Mit
Curated by ChEMBL
Affinity DataIC50: 1nMpH: 7.4 T: 2°CAssay Description:Compounds for testing were diluted in DMSO to 50 fold the final concentration and a ten point three fold dilution series was made. The compounds were...More data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:Inhibition of human recombinant HDAC1 using acetyllysine tripeptide coupled with 7-amino-4-methylcoumarin as substrate by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:Compounds for testing were diluted in DMSO to 50 fold the final concentration and a ten point three fold dilution series was made. The compounds were...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:Inhibition of human recombinant HDAC3 using acetyllysine tripeptide coupled with 7-amino-4-methylcoumarin as substrate by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:Inhibition of human recombinant HDAC3 using acetyllysine tripeptide coupled with 7-amino-4-methylcoumarin as substrate by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:Inhibition of human recombinant HDAC1 using acetyllysine tripeptide coupled with 7-amino-4-methylcoumarin as substrate by fluorescence assayMore data for this Ligand-Target Pair
TargetHistone deacetylase 6(Homo sapiens (Human))
The Broad Institute Of Harvard And Mit
Curated by ChEMBL
The Broad Institute Of Harvard And Mit
Curated by ChEMBL
Affinity DataIC50: 1.5nMpH: 7.4 T: 2°CAssay Description:The compounds were diluted in assay buffer (50 mM HEPES, pH 7.4, 100 mM KCl, 0.001% Tween-20, 0.05% BSA, 20 uM TCEP) to 6 fold their final concentrat...More data for this Ligand-Target Pair
TargetHistone deacetylase 6(Homo sapiens (Human))
The Broad Institute Of Harvard And Mit
Curated by ChEMBL
The Broad Institute Of Harvard And Mit
Curated by ChEMBL
Affinity DataIC50: 1.60nMpH: 7.4 T: 2°CAssay Description:The compounds were diluted in assay buffer (50 mM HEPES, pH 7.4, 100 mM KCl, 0.001% Tween-20, 0.05% BSA, 20 uM TCEP) to 6 fold their final concentrat...More data for this Ligand-Target Pair
Affinity DataIC50: 1.60nMAssay Description:Inhibition of human recombinant HDAC3 using acetyllysine tripeptide coupled with 7-amino-4-methylcoumarin as substrate by fluorescence assayMore data for this Ligand-Target Pair
TargetHistone deacetylase 6(Homo sapiens (Human))
The Broad Institute Of Harvard And Mit
Curated by ChEMBL
The Broad Institute Of Harvard And Mit
Curated by ChEMBL
Affinity DataIC50: 1.70nMpH: 7.4 T: 2°CAssay Description:The compounds were diluted in assay buffer (50 mM HEPES, pH 7.4, 100 mM KCl, 0.001% Tween-20, 0.05% BSA, 20 uM TCEP) to 6 fold their final concentrat...More data for this Ligand-Target Pair
TargetHistone deacetylase 6(Homo sapiens (Human))
The Broad Institute Of Harvard And Mit
Curated by ChEMBL
The Broad Institute Of Harvard And Mit
Curated by ChEMBL
Affinity DataIC50: 1.70nMpH: 7.4 T: 2°CAssay Description:The compounds were diluted in assay buffer (50 mM HEPES, pH 7.4, 100 mM KCl, 0.001% Tween-20, 0.05% BSA, 20 uM TCEP) to 6 fold their final concentrat...More data for this Ligand-Target Pair

 3D Structure (crystal)
3D Structure (crystal)