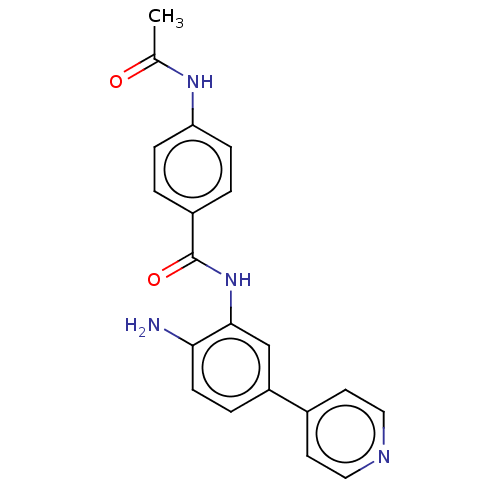
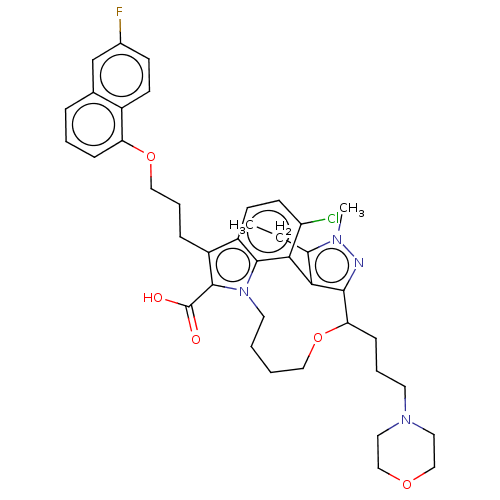
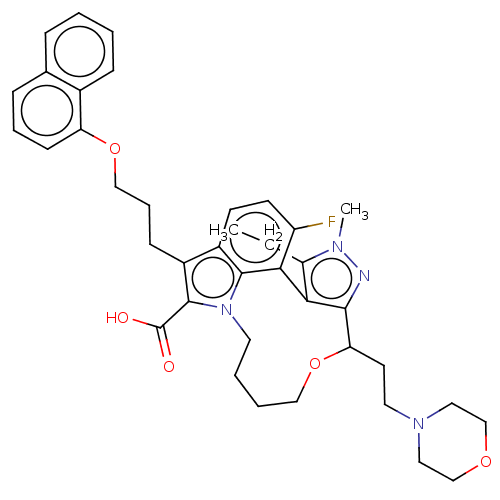
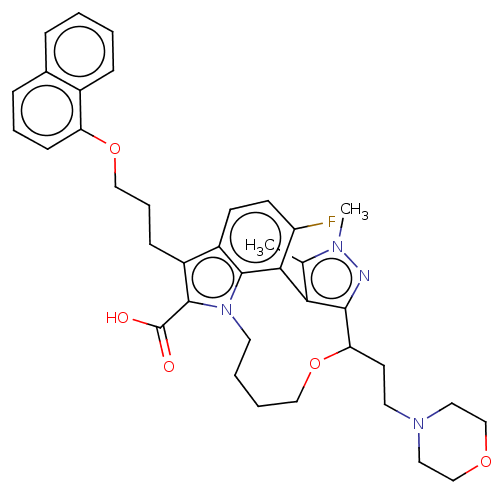
Affinity DataKi: <0.200nM ΔG°: <-55.4kJ/mole IC50: 1nMpH: 7.4 T: 2°CAssay Description:Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ...More data for this Ligand-Target Pair
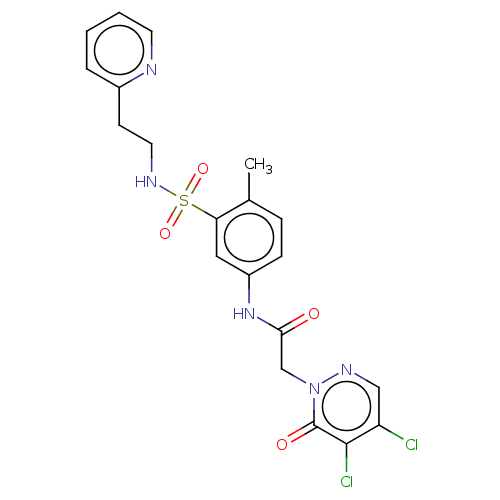
Affinity DataKi: 1.5nM ΔG°: -50.4kJ/mole IC50: 13nMpH: 7.4 T: 2°CAssay Description:Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ...More data for this Ligand-Target Pair
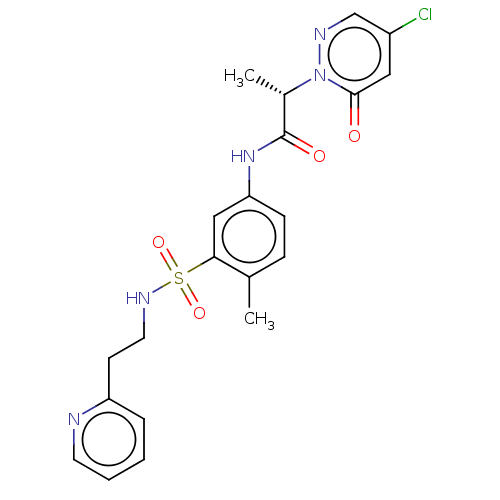
Affinity DataKi: 3nM ΔG°: -48.6kJ/mole IC50: 2nMpH: 7.4 T: 2°CAssay Description:Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ...More data for this Ligand-Target Pair
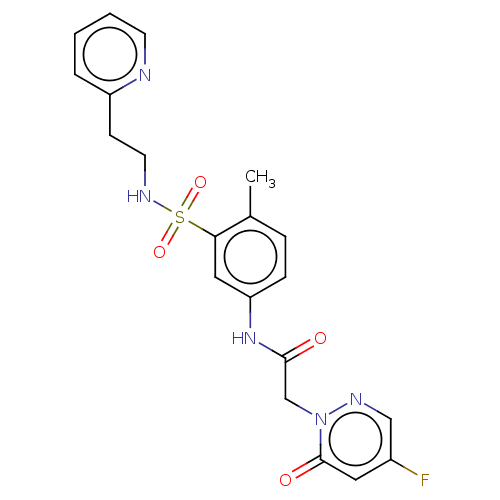
Affinity DataKi: 14nM ΔG°: -44.8kJ/mole IC50: 19nMpH: 7.4 T: 2°CAssay Description:Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ...More data for this Ligand-Target Pair
Affinity DataKi: 25nM ΔG°: -43.4kJ/mole IC50: 46nMpH: 7.4 T: 2°CAssay Description:Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ...More data for this Ligand-Target Pair
Affinity DataKi: 29nM ΔG°: -43.0kJ/mole IC50: 64nMpH: 7.4 T: 2°CAssay Description:Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ...More data for this Ligand-Target Pair
Affinity DataKi: 37nM ΔG°: -42.4kJ/mole IC50: 41nMpH: 7.4 T: 2°CAssay Description:Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ...More data for this Ligand-Target Pair
Affinity DataKi: 223nM ΔG°: -38.0kJ/mole IC50: 147nMpH: 7.4 T: 2°CAssay Description:Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ...More data for this Ligand-Target Pair
Affinity DataKi: 500nM ΔG°: -36.0kJ/mole IC50: 398nMpH: 7.4 T: 2°CAssay Description:Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ...More data for this Ligand-Target Pair
TargetProtein arginine N-methyltransferase 5(Homo sapiens (Human))
The Broad Institute Of Mit And Harvard
Curated by ChEMBL
The Broad Institute Of Mit And Harvard
Curated by ChEMBL
Affinity DataKi: 2.00E+3nMAssay Description:Covalent inhibition of human PRMT5 assessed as initial binding constant by LC-MS analysisMore data for this Ligand-Target Pair
Affinity DataKi: 5.10E+3nM ΔG°: -30.2kJ/mole IC50: 1.08E+3nMpH: 7.4 T: 2°CAssay Description:Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ...More data for this Ligand-Target Pair
Affinity DataKi: 6.30E+3nM ΔG°: -29.7kJ/mole IC50: 1.15E+3nMpH: 7.4 T: 2°CAssay Description:Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ...More data for this Ligand-Target Pair
TargetProtein arginine N-methyltransferase 5(Homo sapiens (Human))
The Broad Institute Of Mit And Harvard
Curated by ChEMBL
The Broad Institute Of Mit And Harvard
Curated by ChEMBL
Affinity DataKi: 8.00E+3nMAssay Description:Covalent inhibition of human PRMT5 assessed as initial binding constant by LC-MS analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1.40E+4nM ΔG°: -27.7kJ/mole IC50: 2.08E+3nMpH: 7.4 T: 2°CAssay Description:Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ...More data for this Ligand-Target Pair
TargetProtein arginine N-methyltransferase 5(Homo sapiens (Human))
The Broad Institute Of Mit And Harvard
Curated by ChEMBL
The Broad Institute Of Mit And Harvard
Curated by ChEMBL
Affinity DataKi: 1.90E+4nMAssay Description:Covalent inhibition of human PRMT5 assessed as initial binding constant by LC-MS analysisMore data for this Ligand-Target Pair
TargetProtein arginine N-methyltransferase 5(Homo sapiens (Human))
The Broad Institute Of Mit And Harvard
Curated by ChEMBL
The Broad Institute Of Mit And Harvard
Curated by ChEMBL
Affinity DataKi: 3.60E+4nMAssay Description:Covalent inhibition of human PRMT5 assessed as initial binding constant by LC-MS analysisMore data for this Ligand-Target Pair
TargetProtein arginine N-methyltransferase 5(Homo sapiens (Human))
The Broad Institute Of Mit And Harvard
Curated by ChEMBL
The Broad Institute Of Mit And Harvard
Curated by ChEMBL
Affinity DataKi: 4.20E+4nMAssay Description:Covalent inhibition of human PRMT5 assessed as initial binding constant by LC-MS analysisMore data for this Ligand-Target Pair
TargetProtein arginine N-methyltransferase 5(Homo sapiens (Human))
The Broad Institute Of Mit And Harvard
Curated by ChEMBL
The Broad Institute Of Mit And Harvard
Curated by ChEMBL
Affinity DataKi: 4.90E+4nMAssay Description:Covalent inhibition of human PRMT5 assessed as initial binding constant by LC-MS analysisMore data for this Ligand-Target Pair
TargetProtein arginine N-methyltransferase 5(Homo sapiens (Human))
The Broad Institute Of Mit And Harvard
Curated by ChEMBL
The Broad Institute Of Mit And Harvard
Curated by ChEMBL
Affinity DataKi: 5.80E+4nMAssay Description:Covalent inhibition of human PRMT5 assessed as initial binding constant by LC-MS analysisMore data for this Ligand-Target Pair
TargetProtein arginine N-methyltransferase 5(Homo sapiens (Human))
The Broad Institute Of Mit And Harvard
Curated by ChEMBL
The Broad Institute Of Mit And Harvard
Curated by ChEMBL
Affinity DataKi: 7.80E+4nMAssay Description:Covalent inhibition of human PRMT5 assessed as initial binding constant by LC-MS analysisMore data for this Ligand-Target Pair
TargetInduced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide(Homo sapiens (Human))
Bayer Aktiengesellschaft
US Patent
Bayer Aktiengesellschaft
US Patent
Affinity DataIC50: 0.260nMAssay Description:The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w...More data for this Ligand-Target Pair
TargetInduced myeloid leukemia cell differentiation protein Mcl-1 [173-329]/Phorbol-Phorbol-12-myristate-13-acetate-induced protein 1 [26-43](Homo sapiens (Human))
The Broad Institute
US Patent
The Broad Institute
US Patent
Affinity DataIC50: 0.310nMAssay Description:MCL-1/Noxa BH3 Peptide (MCL-1 Assay): From there, 50 nl were transferred in a dark test plate (Greiner Bio-One, Frickenhausen, Germany). The assay wa...More data for this Ligand-Target Pair
TargetInduced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide(Homo sapiens (Human))
Bayer Aktiengesellschaft
US Patent
Bayer Aktiengesellschaft
US Patent
Affinity DataIC50: 0.330nMAssay Description:The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w...More data for this Ligand-Target Pair
TargetInduced myeloid leukemia cell differentiation protein Mcl-1 [173-329]/Phorbol-Phorbol-12-myristate-13-acetate-induced protein 1 [26-43](Homo sapiens (Human))
The Broad Institute
US Patent
The Broad Institute
US Patent
Affinity DataIC50: 0.340nMAssay Description:MCL-1/Noxa BH3 Peptide (MCL-1 Assay): From there, 50 nl were transferred in a dark test plate (Greiner Bio-One, Frickenhausen, Germany). The assay wa...More data for this Ligand-Target Pair
TargetInduced myeloid leukemia cell differentiation protein Mcl-1 [173-329]/Phorbol-Phorbol-12-myristate-13-acetate-induced protein 1 [26-43](Homo sapiens (Human))
The Broad Institute
US Patent
The Broad Institute
US Patent
Affinity DataIC50: 0.340nMAssay Description:MCL-1/Noxa BH3 Peptide (MCL-1 Assay): From there, 50 nl were transferred in a dark test plate (Greiner Bio-One, Frickenhausen, Germany). The assay wa...More data for this Ligand-Target Pair
TargetInduced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide(Homo sapiens (Human))
Bayer Aktiengesellschaft
US Patent
Bayer Aktiengesellschaft
US Patent
Affinity DataIC50: 0.370nMAssay Description:The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w...More data for this Ligand-Target Pair
TargetInduced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide(Homo sapiens (Human))
Bayer Aktiengesellschaft
US Patent
Bayer Aktiengesellschaft
US Patent
Affinity DataIC50: 0.370nMAssay Description:The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w...More data for this Ligand-Target Pair
TargetInduced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide(Homo sapiens (Human))
Bayer Aktiengesellschaft
US Patent
Bayer Aktiengesellschaft
US Patent
Affinity DataIC50: 0.390nMAssay Description:The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w...More data for this Ligand-Target Pair
TargetInduced myeloid leukemia cell differentiation protein Mcl-1 [173-329]/Phorbol-Phorbol-12-myristate-13-acetate-induced protein 1 [26-43](Homo sapiens (Human))
The Broad Institute
US Patent
The Broad Institute
US Patent
Affinity DataIC50: 0.400nMAssay Description:MCL-1/Noxa BH3 Peptide (MCL-1 Assay): From there, 50 nl were transferred in a dark test plate (Greiner Bio-One, Frickenhausen, Germany). The assay wa...More data for this Ligand-Target Pair
TargetInduced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide(Homo sapiens (Human))
Bayer Aktiengesellschaft
US Patent
Bayer Aktiengesellschaft
US Patent
Affinity DataIC50: 0.410nMAssay Description:The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w...More data for this Ligand-Target Pair
TargetInduced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide(Homo sapiens (Human))
Bayer Aktiengesellschaft
US Patent
Bayer Aktiengesellschaft
US Patent
Affinity DataIC50: 0.420nMAssay Description:The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w...More data for this Ligand-Target Pair
TargetInduced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide(Homo sapiens (Human))
Bayer Aktiengesellschaft
US Patent
Bayer Aktiengesellschaft
US Patent
Affinity DataIC50: 0.450nMAssay Description:The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w...More data for this Ligand-Target Pair
TargetInduced myeloid leukemia cell differentiation protein Mcl-1(Homo sapiens (Human))
The Broad Institute
US Patent
The Broad Institute
US Patent
Affinity DataIC50: 0.450nMAssay Description:MCL-1 Assay: In the assay 11 different concentrations of each compound (0.1 nM, 0.33 nM, 1.1 nM, 3.8 nM, 13 nM, 44 nM, 0.15 μM, 0.51 μM, 1....More data for this Ligand-Target Pair
TargetInduced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide(Homo sapiens (Human))
Bayer Aktiengesellschaft
US Patent
Bayer Aktiengesellschaft
US Patent
Affinity DataIC50: 0.460nMAssay Description:The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w...More data for this Ligand-Target Pair
TargetInduced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide(Homo sapiens (Human))
Bayer Aktiengesellschaft
US Patent
Bayer Aktiengesellschaft
US Patent
Affinity DataIC50: 0.460nMAssay Description:The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w...More data for this Ligand-Target Pair
TargetInduced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide(Homo sapiens (Human))
Bayer Aktiengesellschaft
US Patent
Bayer Aktiengesellschaft
US Patent
Affinity DataIC50: 0.470nMAssay Description:The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w...More data for this Ligand-Target Pair
TargetInduced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide(Homo sapiens (Human))
Bayer Aktiengesellschaft
US Patent
Bayer Aktiengesellschaft
US Patent
Affinity DataIC50: 0.480nMAssay Description:The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w...More data for this Ligand-Target Pair
TargetInduced myeloid leukemia cell differentiation protein Mcl-1 [173-329]/Phorbol-Phorbol-12-myristate-13-acetate-induced protein 1 [26-43](Homo sapiens (Human))
The Broad Institute
US Patent
The Broad Institute
US Patent
Affinity DataIC50: 0.480nMAssay Description:MCL-1/Noxa BH3 Peptide (MCL-1 Assay): From there, 50 nl were transferred in a dark test plate (Greiner Bio-One, Frickenhausen, Germany). The assay wa...More data for this Ligand-Target Pair
TargetInduced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide(Homo sapiens (Human))
Bayer Aktiengesellschaft
US Patent
Bayer Aktiengesellschaft
US Patent
Affinity DataIC50: 0.480nMAssay Description:The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w...More data for this Ligand-Target Pair
TargetInduced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide(Homo sapiens (Human))
Bayer Aktiengesellschaft
US Patent
Bayer Aktiengesellschaft
US Patent
Affinity DataIC50: 0.490nMAssay Description:The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w...More data for this Ligand-Target Pair
TargetInduced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide(Homo sapiens (Human))
Bayer Aktiengesellschaft
US Patent
Bayer Aktiengesellschaft
US Patent
Affinity DataIC50: 0.520nMAssay Description:The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w...More data for this Ligand-Target Pair
TargetInduced myeloid leukemia cell differentiation protein Mcl-1 [173-329]/Phorbol-Phorbol-12-myristate-13-acetate-induced protein 1 [26-43](Homo sapiens (Human))
The Broad Institute
US Patent
The Broad Institute
US Patent
Affinity DataIC50: 0.520nMAssay Description:MCL-1/Noxa BH3 Peptide (MCL-1 Assay): From there, 50 nl were transferred in a dark test plate (Greiner Bio-One, Frickenhausen, Germany). The assay wa...More data for this Ligand-Target Pair
TargetInduced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide(Homo sapiens (Human))
Bayer Aktiengesellschaft
US Patent
Bayer Aktiengesellschaft
US Patent
Affinity DataIC50: 0.520nMAssay Description:The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w...More data for this Ligand-Target Pair
TargetInduced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide(Homo sapiens (Human))
Bayer Aktiengesellschaft
US Patent
Bayer Aktiengesellschaft
US Patent
Affinity DataIC50: 0.530nMAssay Description:The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w...More data for this Ligand-Target Pair
TargetInduced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide(Homo sapiens (Human))
Bayer Aktiengesellschaft
US Patent
Bayer Aktiengesellschaft
US Patent
Affinity DataIC50: 0.530nMAssay Description:The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w...More data for this Ligand-Target Pair
TargetInduced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide(Homo sapiens (Human))
Bayer Aktiengesellschaft
US Patent
Bayer Aktiengesellschaft
US Patent
Affinity DataIC50: 0.540nMAssay Description:The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w...More data for this Ligand-Target Pair
TargetInduced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide(Homo sapiens (Human))
Bayer Aktiengesellschaft
US Patent
Bayer Aktiengesellschaft
US Patent
Affinity DataIC50: 0.540nMAssay Description:The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w...More data for this Ligand-Target Pair
TargetInduced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide(Homo sapiens (Human))
Bayer Aktiengesellschaft
US Patent
Bayer Aktiengesellschaft
US Patent
Affinity DataIC50: 0.550nMAssay Description:The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w...More data for this Ligand-Target Pair
TargetInduced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide(Homo sapiens (Human))
Bayer Aktiengesellschaft
US Patent
Bayer Aktiengesellschaft
US Patent
Affinity DataIC50: 0.570nMAssay Description:The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w...More data for this Ligand-Target Pair
TargetInduced myeloid leukemia cell differentiation protein Mcl-1(Homo sapiens (Human))
The Broad Institute
US Patent
The Broad Institute
US Patent
Affinity DataIC50: 0.580nMAssay Description:MCL-1 Assay: In the assay 11 different concentrations of each compound (0.1 nM, 0.33 nM, 1.1 nM, 3.8 nM, 13 nM, 44 nM, 0.15 μM, 0.51 μM, 1....More data for this Ligand-Target Pair

 3D Structure (crystal)
3D Structure (crystal)