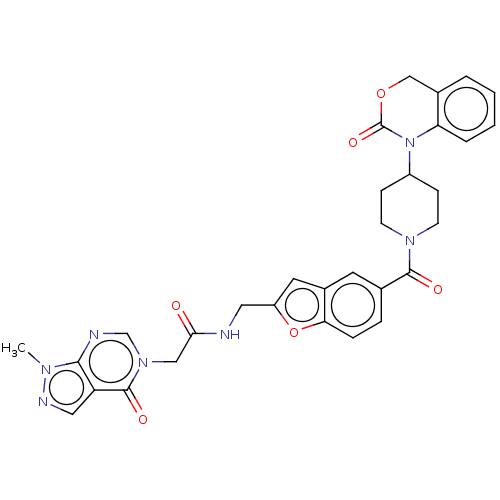
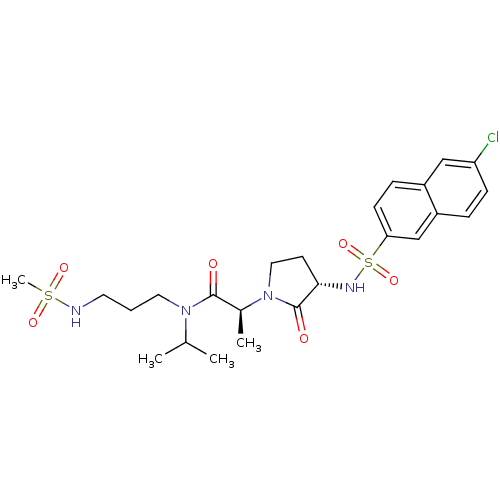
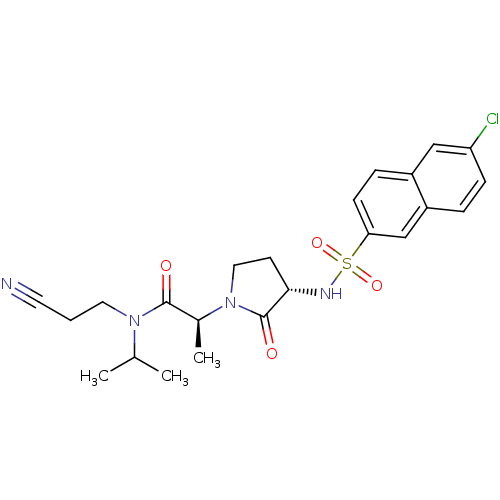
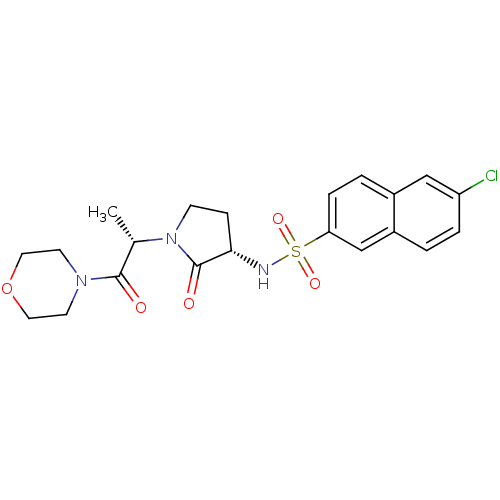
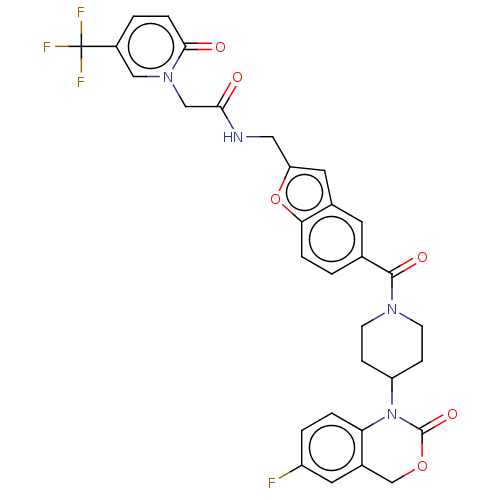
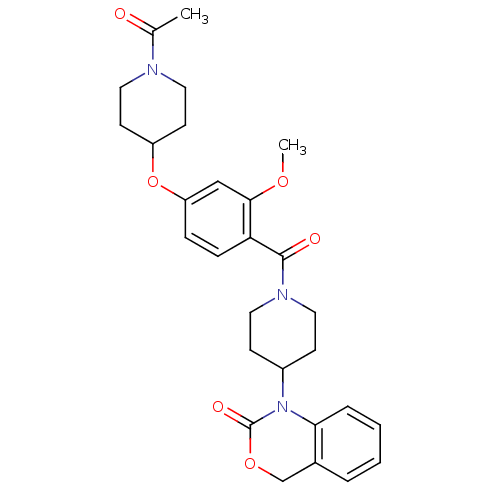
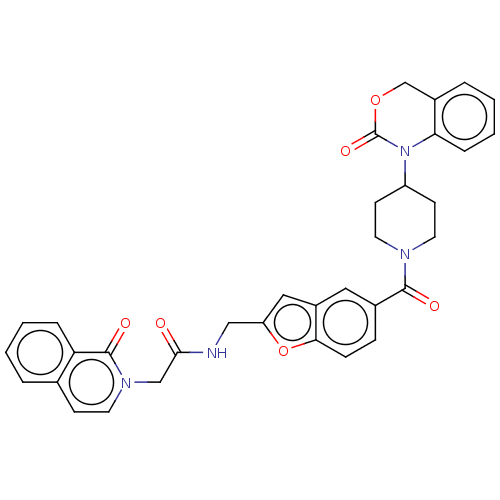
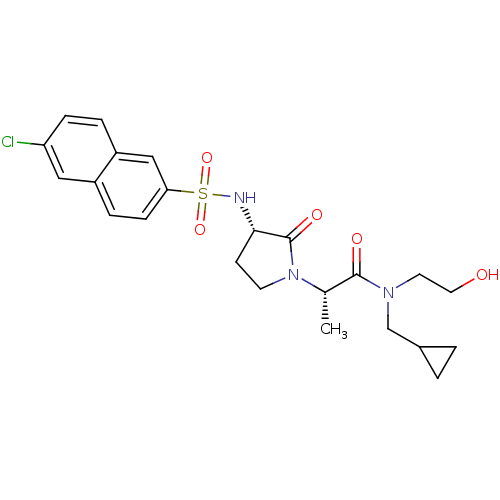
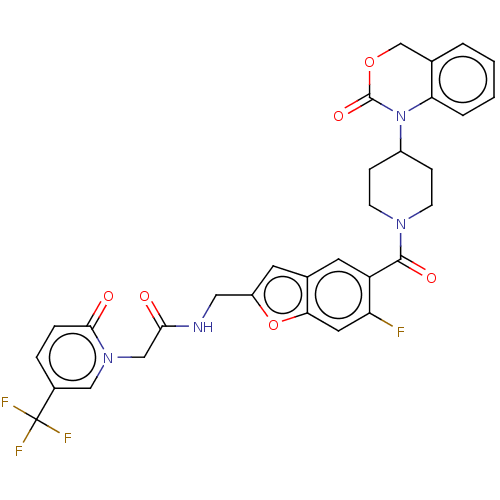
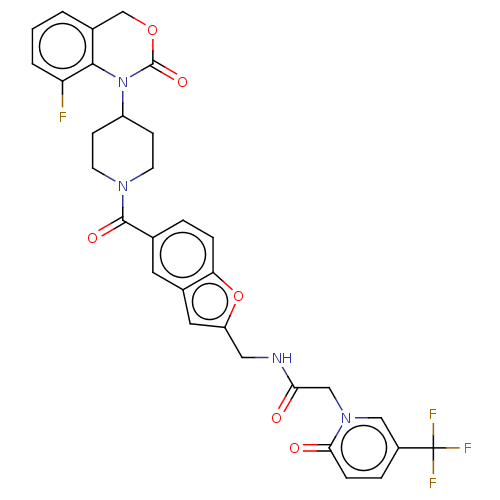
Affinity DataKi: 0.501nMAssay Description:Displacement of 3[H]oxytocin from human oxytocin receptorMore data for this Ligand-Target Pair
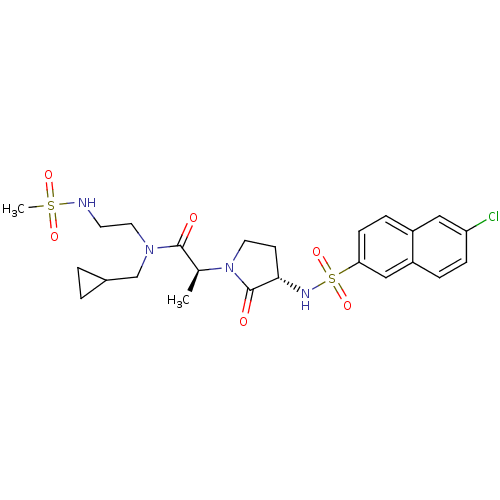
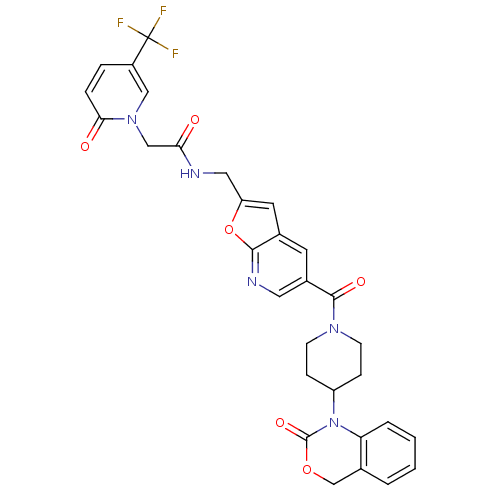
Affinity DataKi: 1nMAssay Description:The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate...More data for this Ligand-Target Pair
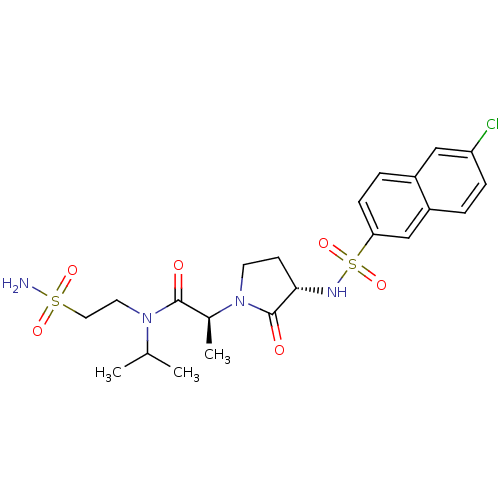
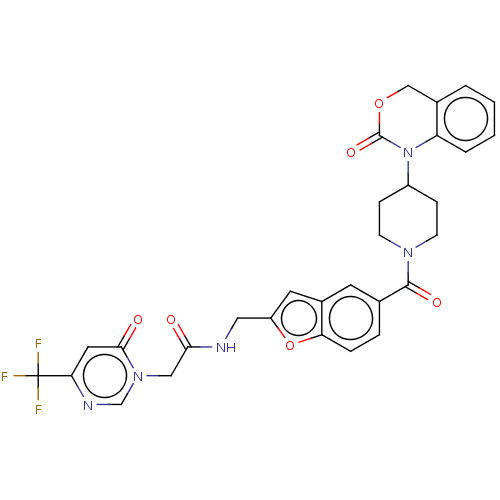
Affinity DataKi: 1nMAssay Description:The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate...More data for this Ligand-Target Pair
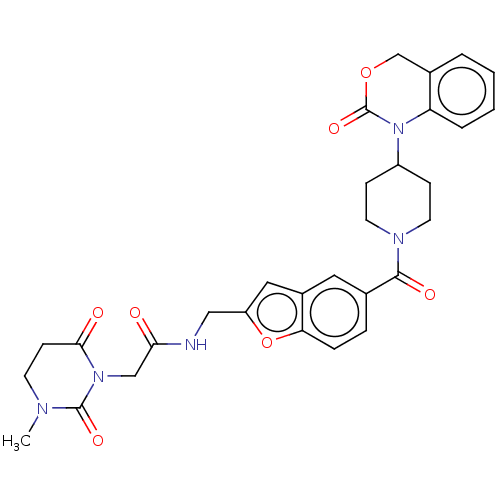
Affinity DataKi: 1nMAssay Description:The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate...More data for this Ligand-Target Pair
Affinity DataKi: <1nMAssay Description:The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate...More data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate...More data for this Ligand-Target Pair
Affinity DataKi: <1nMAssay Description:The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate...More data for this Ligand-Target Pair
Affinity DataKi: <1nMAssay Description:The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate...More data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate...More data for this Ligand-Target Pair
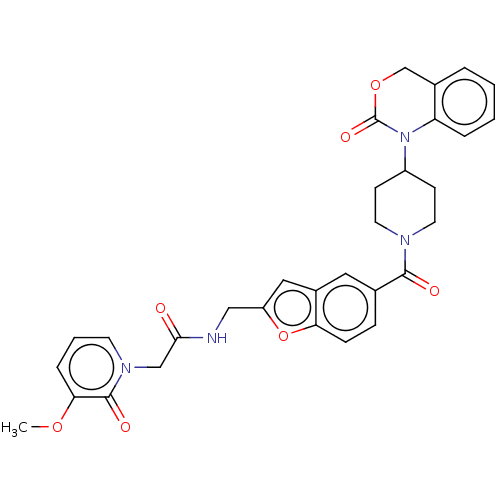
Affinity DataKi: 1nMAssay Description:Displacement of 3[H]oxytocin from human oxytocin receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1.30nMAssay Description:Displacement of 3[H]oxytocin from human oxytocin receptorMore data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate...More data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate...More data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Displacement of 3[H]oxytocin from human oxytocin receptorMore data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Displacement of 3[H]oxytocin from human oxytocin receptorMore data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate...More data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate...More data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate...More data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate...More data for this Ligand-Target Pair
Affinity DataKi: 2.5nMAssay Description:Displacement of 3[H]oxytocin from human oxytocin receptorMore data for this Ligand-Target Pair
Affinity DataKi: 3nM ΔG°: -48.6kJ/molepH: 7.8 T: 2°CAssay Description:The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate...More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate...More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate...More data for this Ligand-Target Pair
Affinity DataKi: 3.20nMAssay Description:Displacement of 3[H]oxytocin from human oxytocin receptorMore data for this Ligand-Target Pair
Affinity DataKi: 3.20nMAssay Description:Displacement of 3[H]oxytocin from human oxytocin receptorMore data for this Ligand-Target Pair
Affinity DataKi: 3.20nMAssay Description:Ability to displace [3H]oxytocin from human OT receptor (hOT)More data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Displacement of 3[H]oxytocin from human oxytocin receptorMore data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Ability to displace [3H]oxytocin from human OT receptor (hOT)More data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate...More data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate...More data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate...More data for this Ligand-Target Pair
Affinity DataKi: 5nM ΔG°: -47.4kJ/molepH: 7.8 T: 2°CAssay Description:The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate...More data for this Ligand-Target Pair
Affinity DataKi: 6nMAssay Description:The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate...More data for this Ligand-Target Pair
Affinity DataKi: 6nMAssay Description:The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate...More data for this Ligand-Target Pair
Affinity DataKi: 6nMAssay Description:The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate...More data for this Ligand-Target Pair
Affinity DataKi: 6.30nMAssay Description:Ability to displace [3H]oxytocin from human OT receptor (hOT)More data for this Ligand-Target Pair
Affinity DataKi: 6.30nMAssay Description:Displacement of 3[H]oxytocin from human oxytocin receptorMore data for this Ligand-Target Pair
Affinity DataKi: 6.30nMAssay Description:Ability to displace [3H]oxytocin from human OT receptor (hOT)More data for this Ligand-Target Pair
Affinity DataKi: 6.30nMAssay Description:Displacement of 3[H]oxytocin from human oxytocin receptorMore data for this Ligand-Target Pair
Affinity DataKi: 6.30nMAssay Description:Displacement of 3[H]oxytocin from human oxytocin receptorMore data for this Ligand-Target Pair
Affinity DataKi: 6.30nMAssay Description:Displacement of 3[H]oxytocin from human oxytocin receptorMore data for this Ligand-Target Pair
Affinity DataKi: 6.30nMAssay Description:Displacement of 3[H]oxytocin from human oxytocin receptorMore data for this Ligand-Target Pair
Affinity DataKi: 7nMAssay Description:The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate...More data for this Ligand-Target Pair
Affinity DataKi: 7nMAssay Description:The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate...More data for this Ligand-Target Pair
Affinity DataKi: 7.90nMAssay Description:Displacement of 3[H]oxytocin from human oxytocin receptorMore data for this Ligand-Target Pair
Affinity DataKi: 7.90nMAssay Description:Displacement of 3[H]oxytocin from human oxytocin receptorMore data for this Ligand-Target Pair
Affinity DataKi: 9nMAssay Description:The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate...More data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:Displacement of 3[H]oxytocin from human oxytocin receptorMore data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:Ability to displace [3H]oxytocin from human OT receptor (hOT)More data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:Displacement of 3[H]oxytocin from human oxytocin receptorMore data for this Ligand-Target Pair








 3D Structure (crystal)
3D Structure (crystal)