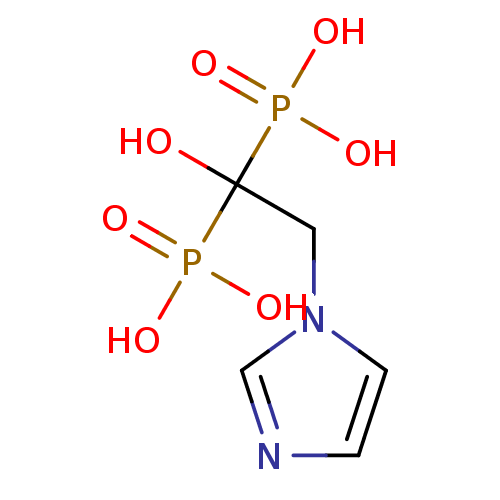
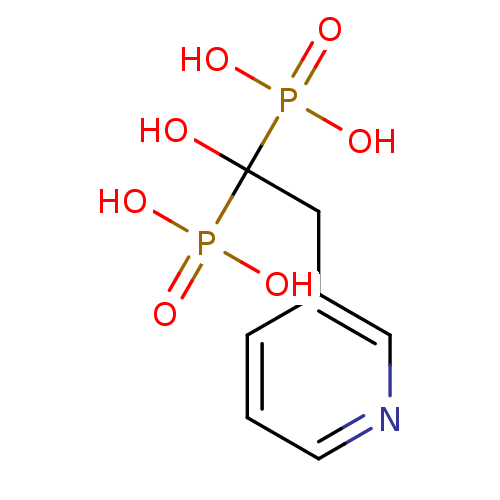
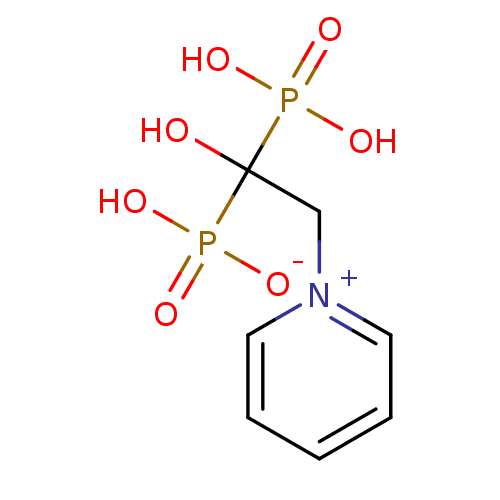
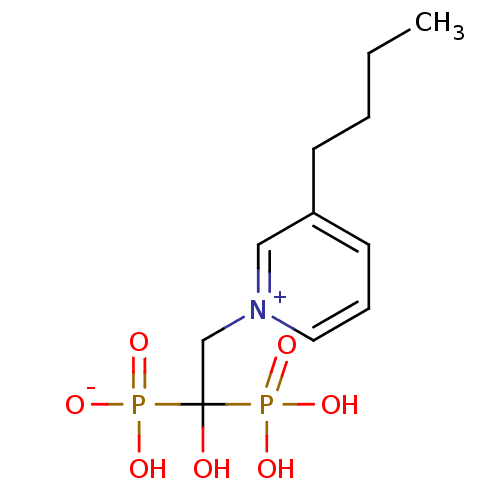
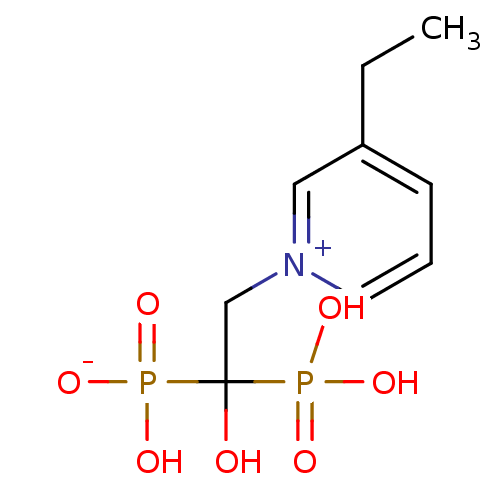
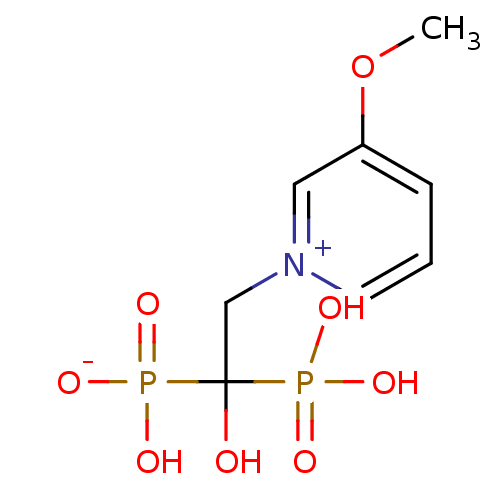
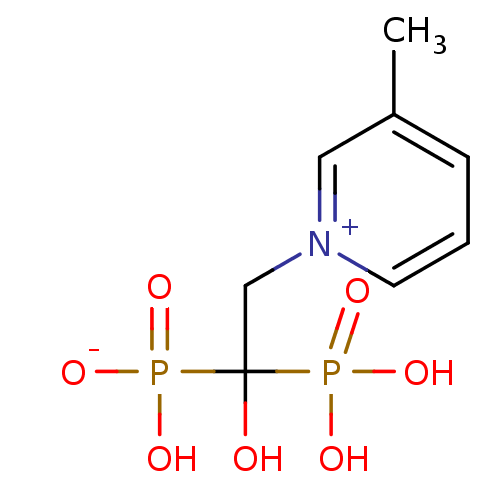
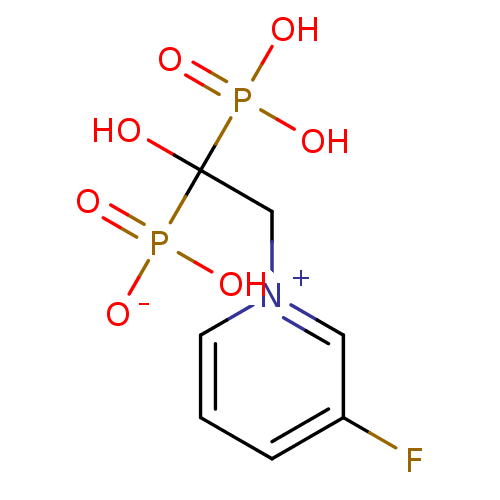
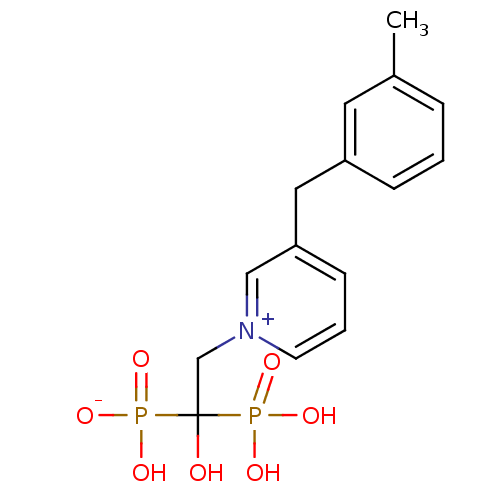
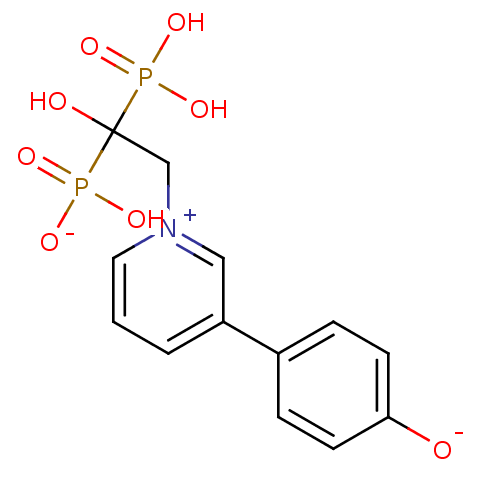
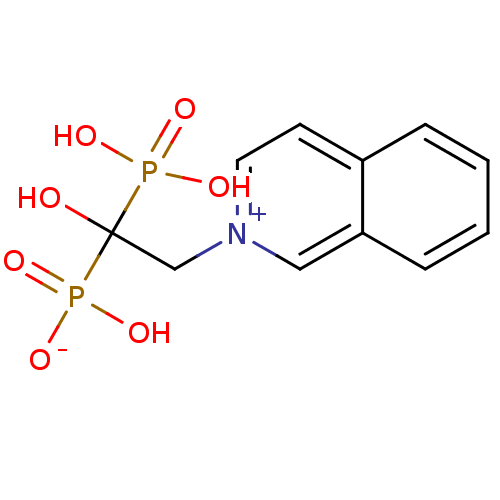
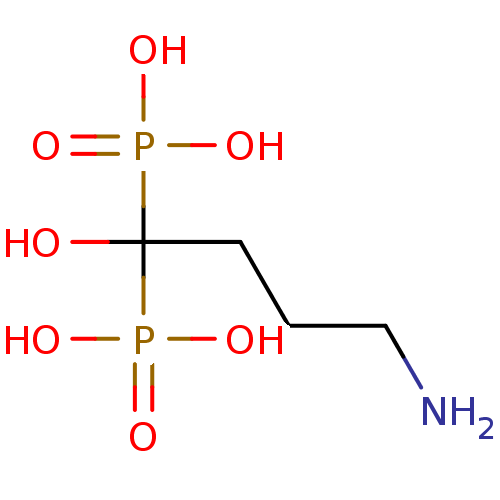
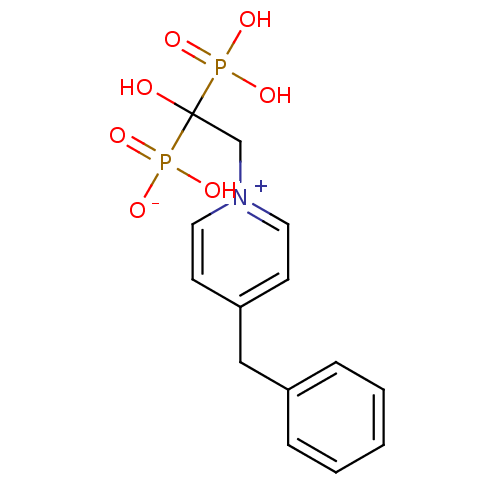
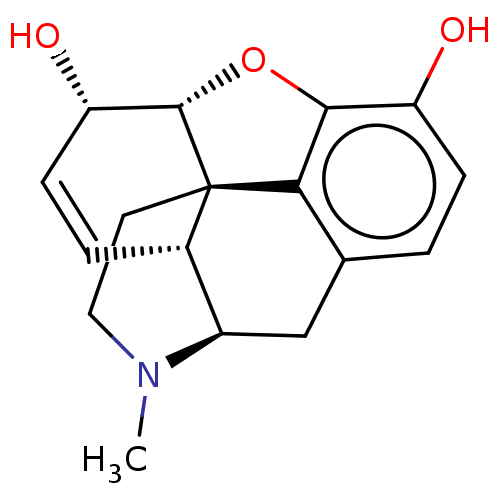
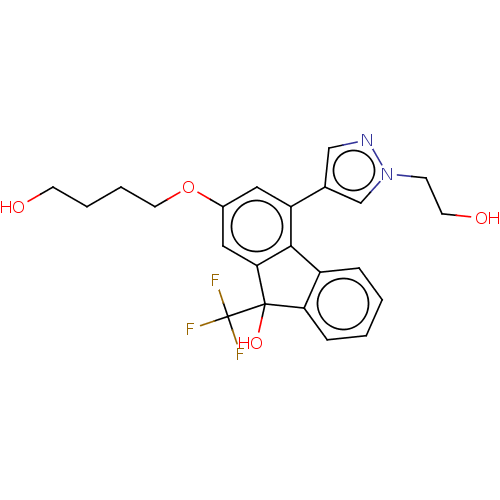
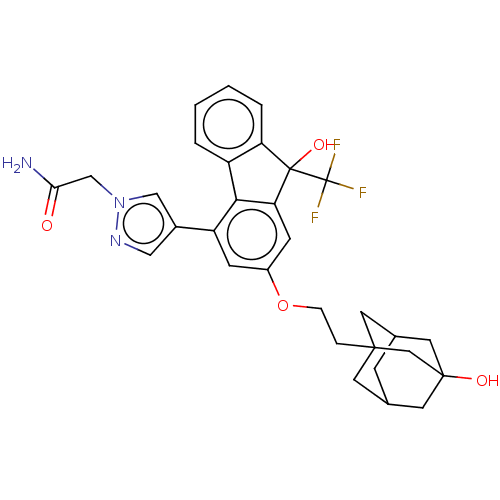
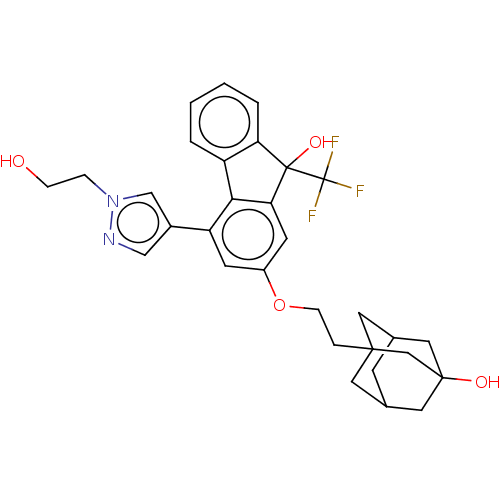
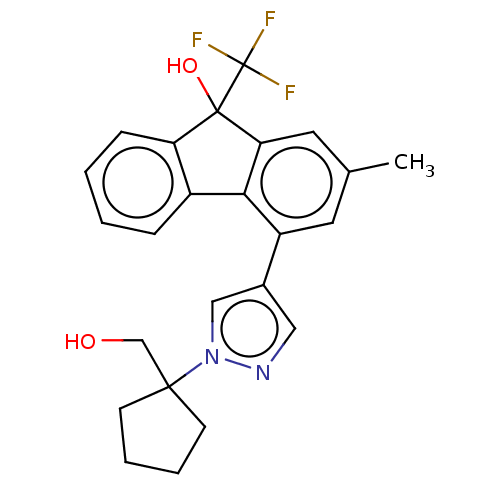
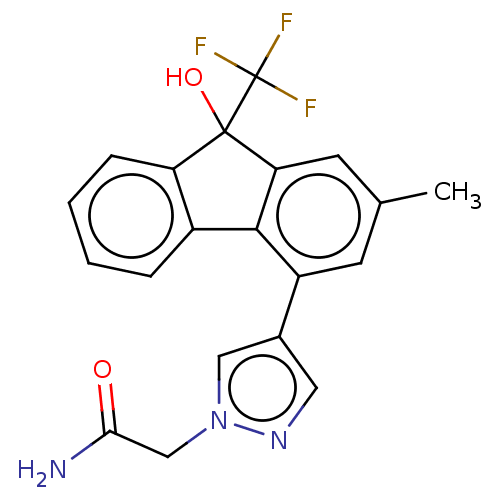
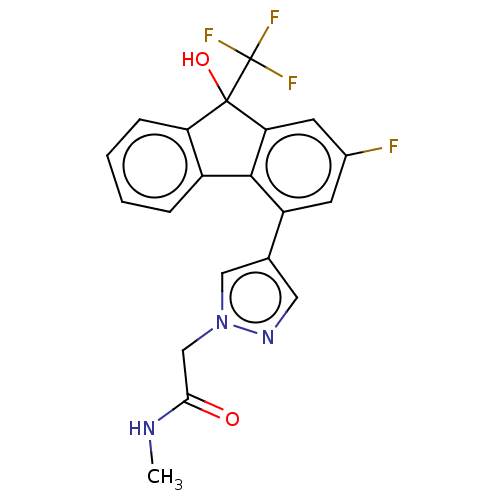
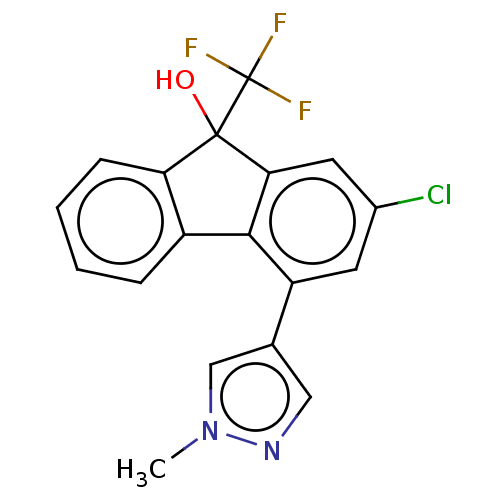
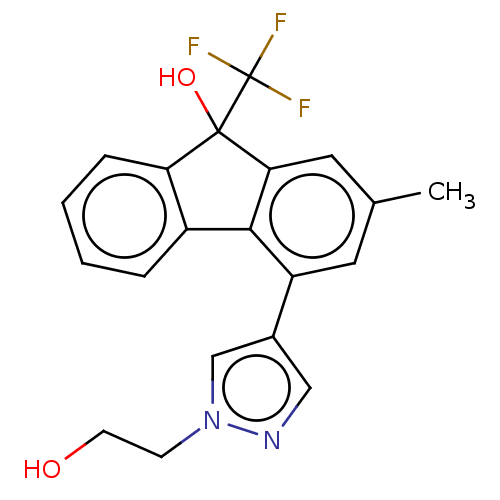
Target4,4'-diapophytoene synthase(Staphylococcus aureus)
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
Affinity DataKi: 0.5nMAssay Description:Inhibition of Staphylococcus aureus dehydrosqualene synthase expressed in Escherichia coli BL21 (DE3) cells by continuous spectrophotometric assayMore data for this Ligand-Target Pair
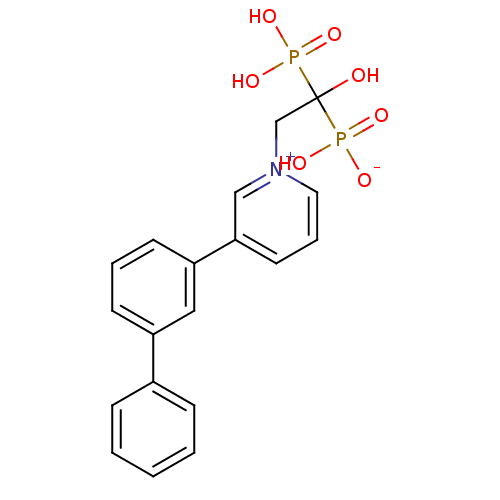
Target4,4'-diapophytoene synthase(Staphylococcus aureus)
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:Inhibition of Staphylococcus aureus dehydrosqualene synthase expressed in Escherichia coli BL21 (DE3) cells by continuous spectrophotometric assayMore data for this Ligand-Target Pair
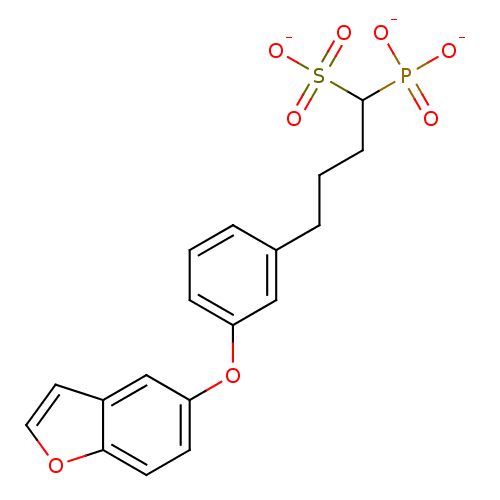
Target4,4'-diapophytoene synthase(Staphylococcus aureus)
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
Affinity DataKi: 5nMAssay Description:Inhibition of Staphylococcus aureus dehydrosqualene synthase expressed in Escherichia coli BL21 (DE3) cells by continuous spectrophotometric assayMore data for this Ligand-Target Pair
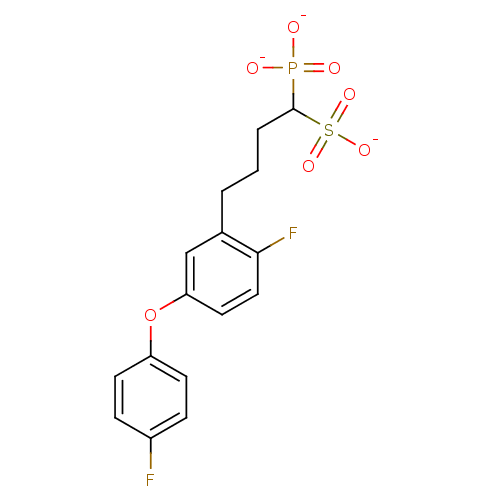
Target4,4'-diapophytoene synthase(Staphylococcus aureus)
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
Affinity DataKi: 5nMAssay Description:Inhibition of Staphylococcus aureus dehydrosqualene synthase expressed in Escherichia coli BL21 (DE3) cells by continuous spectrophotometric assayMore data for this Ligand-Target Pair
TargetPolyprenyl synthetase family protein(Plasmodium falciparum (isolate 3D7))
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
Affinity DataKi: 9nMAssay Description:Binding affinity towards Farnesyl diphosphate synthase from leishmania majorMore data for this Ligand-Target Pair
TargetPolyprenyl synthetase family protein(Plasmodium falciparum (isolate 3D7))
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
Affinity DataKi: 11nMAssay Description:Binding affinity towards Farnesyl diphosphate synthase from leishmania majorMore data for this Ligand-Target Pair
TargetPolyprenyl synthetase family protein(Plasmodium falciparum (isolate 3D7))
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
Affinity DataKi: 17nMAssay Description:Binding affinity towards Farnesyl diphosphate synthase from leishmania majorMore data for this Ligand-Target Pair
TargetPolyprenyl synthetase family protein(Plasmodium falciparum (isolate 3D7))
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
Affinity DataKi: 18nMAssay Description:Binding affinity towards Farnesyl diphosphate synthase from leishmania majorMore data for this Ligand-Target Pair
TargetPolyprenyl synthetase family protein(Plasmodium falciparum (isolate 3D7))
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
Affinity DataKi: 20nMAssay Description:Binding affinity towards Farnesyl diphosphate synthase from leishmania majorMore data for this Ligand-Target Pair
TargetPolyprenyl synthetase family protein(Plasmodium falciparum (isolate 3D7))
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
Affinity DataKi: 20nMAssay Description:Binding affinity towards Farnesyl diphosphate synthase from leishmania majorMore data for this Ligand-Target Pair
TargetPolyprenyl synthetase family protein(Plasmodium falciparum (isolate 3D7))
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
Affinity DataKi: 30nMAssay Description:Binding affinity towards Farnesyl diphosphate synthase from leishmania majorMore data for this Ligand-Target Pair
TargetPolyprenyl synthetase family protein(Plasmodium falciparum (isolate 3D7))
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
Affinity DataKi: 38nMAssay Description:Binding affinity towards Farnesyl diphosphate synthase from leishmania majorMore data for this Ligand-Target Pair
TargetPolyprenyl synthetase family protein(Plasmodium falciparum (isolate 3D7))
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
Affinity DataKi: 50nMAssay Description:Binding affinity towards Farnesyl diphosphate synthase from leishmania majorMore data for this Ligand-Target Pair
TargetPolyprenyl synthetase family protein(Plasmodium falciparum (isolate 3D7))
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
Affinity DataKi: 70nMAssay Description:Binding affinity towards Farnesyl diphosphate synthase from leishmania majorMore data for this Ligand-Target Pair
TargetPolyprenyl synthetase family protein(Plasmodium falciparum (isolate 3D7))
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
Affinity DataKi: 75nMAssay Description:Binding affinity towards Farnesyl diphosphate synthase from leishmania majorMore data for this Ligand-Target Pair
TargetPolyprenyl synthetase family protein(Plasmodium falciparum (isolate 3D7))
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
Affinity DataKi: 80nMAssay Description:Binding affinity towards Farnesyl diphosphate synthase from leishmania majorMore data for this Ligand-Target Pair
TargetPolyprenyl synthetase family protein(Plasmodium falciparum (isolate 3D7))
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
Affinity DataKi: 95nMAssay Description:Binding affinity towards Farnesyl diphosphate synthase from leishmania majorMore data for this Ligand-Target Pair
TargetPolyprenyl synthetase family protein(Plasmodium falciparum (isolate 3D7))
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
Affinity DataKi: 110nMAssay Description:Binding affinity towards Farnesyl diphosphate synthase from leishmania majorMore data for this Ligand-Target Pair
TargetPolyprenyl synthetase family protein(Plasmodium falciparum (isolate 3D7))
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
Affinity DataKi: 160nMAssay Description:Binding affinity towards Farnesyl diphosphate synthase from leishmania majorMore data for this Ligand-Target Pair
TargetPolyprenyl synthetase family protein(Plasmodium falciparum (isolate 3D7))
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
Affinity DataKi: 190nMAssay Description:Binding affinity towards Farnesyl diphosphate synthase from leishmania majorMore data for this Ligand-Target Pair
TargetPolyprenyl synthetase family protein(Plasmodium falciparum (isolate 3D7))
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
Affinity DataKi: 380nMAssay Description:Binding affinity towards Farnesyl diphosphate synthase from leishmania majorMore data for this Ligand-Target Pair
TargetPolyprenyl synthetase family protein(Plasmodium falciparum (isolate 3D7))
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
Affinity DataKi: 950nMAssay Description:Binding affinity towards Farnesyl diphosphate synthase from leishmania majorMore data for this Ligand-Target Pair
TargetSqualene synthase(Homo sapiens (Human))
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of human recombinant squalene synthase expressed in Escherichia coli BL21 (DE3) cells assessed as formation of 1,10-dioic acid metabolite ...More data for this Ligand-Target Pair
TargetSqualene synthase(Homo sapiens (Human))
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of human recombinant squalene synthase expressed in Escherichia coli BL21 (DE3) cells assessed as formation of 1,10-dioic acid metabolite ...More data for this Ligand-Target Pair
TargetSqualene synthase(Homo sapiens (Human))
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of human recombinant squalene synthase expressed in Escherichia coli BL21 (DE3) cells assessed as formation of 1,10-dioic acid metabolite ...More data for this Ligand-Target Pair
TargetSqualene synthase(Homo sapiens (Human))
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of human recombinant squalene synthase expressed in Escherichia coli BL21 (DE3) cells assessed as formation of 1,10-dioic acid metabolite ...More data for this Ligand-Target Pair
TargetSqualene synthase(Homo sapiens (Human))
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of human recombinant squalene synthase expressed in Escherichia coli BL21 (DE3) cells assessed as formation of 1,10-dioic acid metabolite ...More data for this Ligand-Target Pair
TargetSqualene synthase(Homo sapiens (Human))
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of human recombinant squalene synthase expressed in Escherichia coli BL21 (DE3) cells assessed as formation of 1,10-dioic acid metabolite ...More data for this Ligand-Target Pair
TargetSqualene synthase(Homo sapiens (Human))
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
Affinity DataIC50: 8nMAssay Description:Inhibition of human recombinant squalene synthase expressed in Escherichia coli BL21 (DE3) cells assessed as formation of 1,10-dioic acid metabolite ...More data for this Ligand-Target Pair
TargetDelta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1(Rattus norvegicus (rat))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 8.60nMAssay Description:Inhibitory activity against opiate receptor in rat using [3H]naloxone as radioligandMore data for this Ligand-Target Pair
TargetSqualene synthase(Homo sapiens (Human))
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
Affinity DataIC50: 14nMAssay Description:Inhibition of human recombinant squalene synthase expressed in Escherichia coli BL21 (DE3) cells assessed as formation of 1,10-dioic acid metabolite ...More data for this Ligand-Target Pair
Target[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 2, mitochondrial(Homo sapiens (Human))
Japan Tobacco
US Patent
Japan Tobacco
US Patent
Affinity DataIC50: 14nMpH: 8.0 T: 2°CAssay Description:The inhibitory action of PDHK activity was indirectly evaluated by performing a kinase reaction in the presence of a test compound and measuring the ...More data for this Ligand-Target Pair
Target[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 2, mitochondrial(Homo sapiens (Human))
Japan Tobacco
US Patent
Japan Tobacco
US Patent
Affinity DataIC50: 15nMpH: 8.0 T: 2°CAssay Description:The inhibitory action of PDHK activity was indirectly evaluated by performing a kinase reaction in the presence of a test compound and measuring the ...More data for this Ligand-Target Pair
Target[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 2, mitochondrial(Homo sapiens (Human))
Japan Tobacco
US Patent
Japan Tobacco
US Patent
Affinity DataIC50: 15nMpH: 8.0 T: 2°CAssay Description:The inhibitory action of PDHK activity was indirectly evaluated by performing a kinase reaction in the presence of a test compound and measuring the ...More data for this Ligand-Target Pair
Target[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 2, mitochondrial(Homo sapiens (Human))
Japan Tobacco
US Patent
Japan Tobacco
US Patent
Affinity DataIC50: 15nMpH: 8.0 T: 2°CAssay Description:The inhibitory action of PDHK activity was indirectly evaluated by performing a kinase reaction in the presence of a test compound and measuring the ...More data for this Ligand-Target Pair
Target[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 2, mitochondrial(Homo sapiens (Human))
Japan Tobacco
US Patent
Japan Tobacco
US Patent
Affinity DataIC50: 15nMpH: 8.0 T: 2°CAssay Description:The inhibitory action of PDHK activity was indirectly evaluated by performing a kinase reaction in the presence of a test compound and measuring the ...More data for this Ligand-Target Pair
Target[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 2, mitochondrial(Homo sapiens (Human))
Japan Tobacco
US Patent
Japan Tobacco
US Patent
Affinity DataIC50: 15nMpH: 8.0 T: 2°CAssay Description:The inhibitory action of PDHK activity was indirectly evaluated by performing a kinase reaction in the presence of a test compound and measuring the ...More data for this Ligand-Target Pair
Target[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 2, mitochondrial(Homo sapiens (Human))
Japan Tobacco
US Patent
Japan Tobacco
US Patent
Affinity DataIC50: 15nMpH: 8.0 T: 2°CAssay Description:The inhibitory action of PDHK activity was indirectly evaluated by performing a kinase reaction in the presence of a test compound and measuring the ...More data for this Ligand-Target Pair
Target[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 2, mitochondrial(Homo sapiens (Human))
Japan Tobacco
US Patent
Japan Tobacco
US Patent
Affinity DataIC50: 16nMpH: 8.0 T: 2°CAssay Description:The inhibitory action of PDHK activity was indirectly evaluated by performing a kinase reaction in the presence of a test compound and measuring the ...More data for this Ligand-Target Pair
Target[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 2, mitochondrial(Homo sapiens (Human))
Japan Tobacco
US Patent
Japan Tobacco
US Patent
Affinity DataIC50: 16nMpH: 8.0 T: 2°CAssay Description:The inhibitory action of PDHK activity was indirectly evaluated by performing a kinase reaction in the presence of a test compound and measuring the ...More data for this Ligand-Target Pair
Target[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 2, mitochondrial(Homo sapiens (Human))
Japan Tobacco
US Patent
Japan Tobacco
US Patent
Affinity DataIC50: 16nMpH: 8.0 T: 2°CAssay Description:The inhibitory action of PDHK activity was indirectly evaluated by performing a kinase reaction in the presence of a test compound and measuring the ...More data for this Ligand-Target Pair
Target[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 2, mitochondrial(Homo sapiens (Human))
Japan Tobacco
US Patent
Japan Tobacco
US Patent
Affinity DataIC50: 16nMpH: 8.0 T: 2°CAssay Description:The inhibitory action of PDHK activity was indirectly evaluated by performing a kinase reaction in the presence of a test compound and measuring the ...More data for this Ligand-Target Pair
Target[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 2, mitochondrial(Homo sapiens (Human))
Japan Tobacco
US Patent
Japan Tobacco
US Patent
Affinity DataIC50: 16nMpH: 8.0 T: 2°CAssay Description:The inhibitory action of PDHK activity was indirectly evaluated by performing a kinase reaction in the presence of a test compound and measuring the ...More data for this Ligand-Target Pair
TargetSqualene synthase(Homo sapiens (Human))
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
Affinity DataIC50: 17nMAssay Description:Inhibition of human recombinant squalene synthase expressed in Escherichia coli BL21 (DE3) cells assessed as formation of 1,10-dioic acid metabolite ...More data for this Ligand-Target Pair
Target[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 2, mitochondrial(Homo sapiens (Human))
Japan Tobacco
US Patent
Japan Tobacco
US Patent
Affinity DataIC50: 17nMpH: 8.0 T: 2°CAssay Description:The inhibitory action of PDHK activity was indirectly evaluated by performing a kinase reaction in the presence of a test compound and measuring the ...More data for this Ligand-Target Pair
Target[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 2, mitochondrial(Homo sapiens (Human))
Japan Tobacco
US Patent
Japan Tobacco
US Patent
Affinity DataIC50: 17nMpH: 8.0 T: 2°CAssay Description:The inhibitory action of PDHK activity was indirectly evaluated by performing a kinase reaction in the presence of a test compound and measuring the ...More data for this Ligand-Target Pair
Target[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 2, mitochondrial(Homo sapiens (Human))
Japan Tobacco
US Patent
Japan Tobacco
US Patent
Affinity DataIC50: 17nMpH: 8.0 T: 2°CAssay Description:The inhibitory action of PDHK activity was indirectly evaluated by performing a kinase reaction in the presence of a test compound and measuring the ...More data for this Ligand-Target Pair
Target[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 2, mitochondrial(Homo sapiens (Human))
Japan Tobacco
US Patent
Japan Tobacco
US Patent
Affinity DataIC50: 17nMpH: 8.0 T: 2°CAssay Description:The inhibitory action of PDHK activity was indirectly evaluated by performing a kinase reaction in the presence of a test compound and measuring the ...More data for this Ligand-Target Pair
Target[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 2, mitochondrial(Homo sapiens (Human))
Japan Tobacco
US Patent
Japan Tobacco
US Patent
Affinity DataIC50: 17nMpH: 8.0 T: 2°CAssay Description:The inhibitory action of PDHK activity was indirectly evaluated by performing a kinase reaction in the presence of a test compound and measuring the ...More data for this Ligand-Target Pair
Target[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 2, mitochondrial(Homo sapiens (Human))
Japan Tobacco
US Patent
Japan Tobacco
US Patent
Affinity DataIC50: 17nMpH: 8.0 T: 2°CAssay Description:The inhibitory action of PDHK activity was indirectly evaluated by performing a kinase reaction in the presence of a test compound and measuring the ...More data for this Ligand-Target Pair



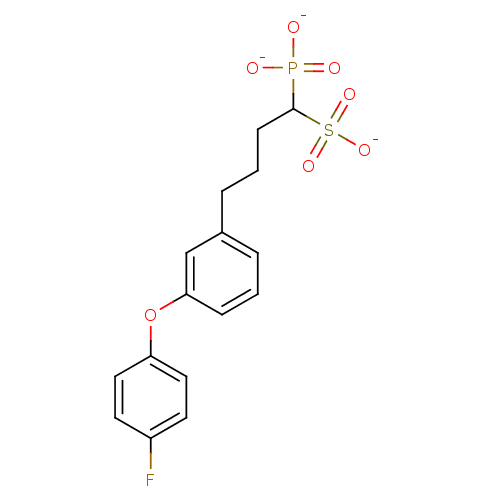


 3D Structure (crystal)
3D Structure (crystal)