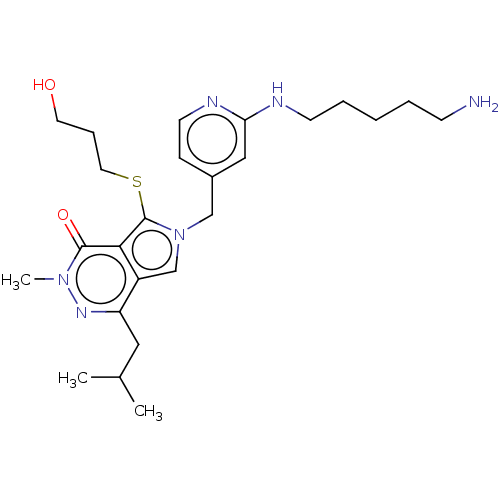
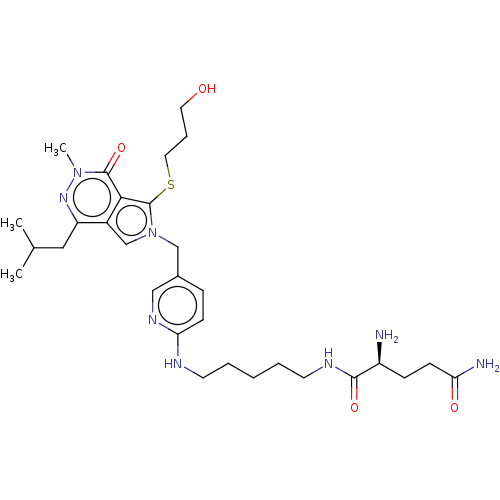
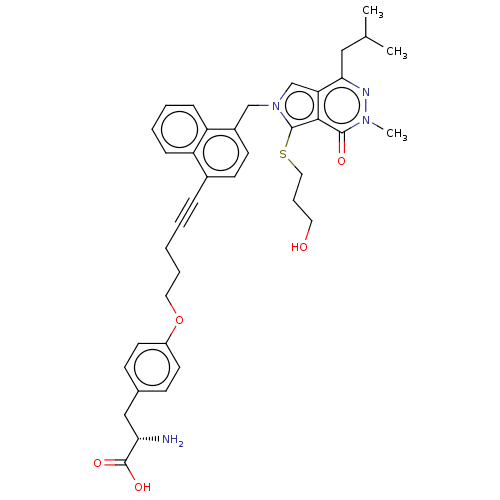
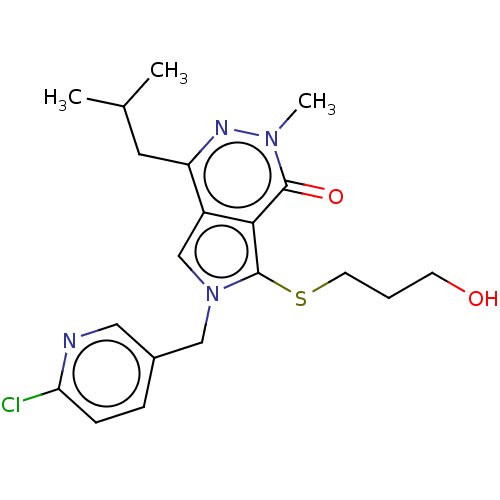
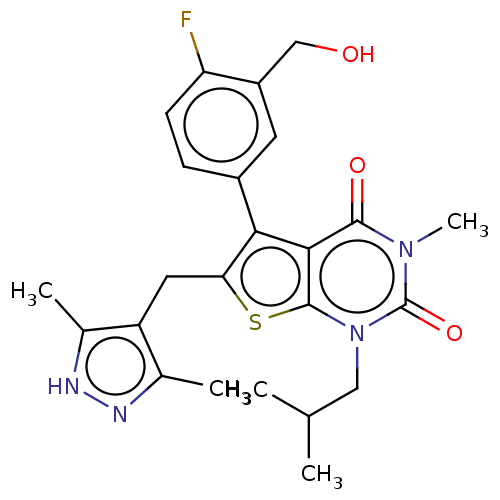
Affinity DataIC50: 2.90nMAssay Description:Compound was evaluated for its inhibitory activity against Isoleucyl-tRNA synthetase from rat liverMore data for this Ligand-Target Pair
Affinity DataIC50: 4.20nMAssay Description:Compound was evaluated for its inhibitory activity against Isoleucyl-tRNA synthetase from rat liverMore data for this Ligand-Target Pair
Affinity DataIC50: 14nMAssay Description:Compound was evaluated for its inhibitory activity against Isoleucyl-tRNA synthetase from staphylococcus aureus WCUH29More data for this Ligand-Target Pair
TargetLeucine--tRNA ligase, cytoplasmic(Homo sapiens (Human))
Australian National University
Curated by ChEMBL
Australian National University
Curated by ChEMBL
Affinity DataIC50: 16nMAssay Description:Compound was evaluated for its inhibitory activity against Leucyl-tRNA synthetase from staphylococcus aureus WCUH29More data for this Ligand-Target Pair
Affinity DataIC50: 17nMAssay Description:Compound was evaluated for its inhibitory activity against Isoleucyl-tRNA synthetase from rat liverMore data for this Ligand-Target Pair
Affinity DataIC50: 25nMAssay Description:Compound was evaluated for its inhibitory activity against Isoleucyl-tRNA synthetase from rat liverMore data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Compound was evaluated for its inhibitory activity against VRS (valyl tRNA synthetase) from staphylococcus aureus WCUH29More data for this Ligand-Target Pair
Affinity DataIC50: 37nMAssay Description:Compound was evaluated for its inhibitory activity against Isoleucyl-tRNA synthetase from staphylococcus aureus WCUH29More data for this Ligand-Target Pair
Affinity DataIC50: 60nMAssay Description:Compound was evaluated for its inhibitory activity against Isoleucyl-tRNA synthetase from staphylococcus aureus WCUH29More data for this Ligand-Target Pair
Affinity DataIC50: 68nMAssay Description:Compound was evaluated for its inhibitory activity against Isoleucyl-tRNA synthetase from rat liverMore data for this Ligand-Target Pair
Affinity DataIC50: 120nMAssay Description:Compound was evaluated for its inhibitory activity against Isoleucyl-tRNA synthetase from staphylococcus aureus WCUH29More data for this Ligand-Target Pair
Affinity DataIC50: 126nMAssay Description:Compound was evaluated for its inhibitory activity against VRS (valyl tRNA synthetase) from staphylococcus aureus WCUH29More data for this Ligand-Target Pair
Affinity DataIC50: 290nMAssay Description:Compound was evaluated for its inhibitory activity against VRS (valyl tRNA synthetase) from staphylococcus aureus WCUH29More data for this Ligand-Target Pair
Affinity DataIC50: 500nMAssay Description:Compound was evaluated for its inhibitory activity against Isoleucyl-tRNA synthetase from rat liverMore data for this Ligand-Target Pair
Affinity DataIC50: 910nMAssay Description:Compound was evaluated for its inhibitory activity against Isoleucyl-tRNA synthetase from staphylococcus aureus WCUH29More data for this Ligand-Target Pair
TargetLeucine--tRNA ligase, cytoplasmic(Homo sapiens (Human))
Australian National University
Curated by ChEMBL
Australian National University
Curated by ChEMBL
Affinity DataIC50: 1.55E+3nMAssay Description:Compound was evaluated for its inhibitory activity against Leucyl-tRNA synthetase from staphylococcus aureus WCUH29More data for this Ligand-Target Pair
TargetLeucine--tRNA ligase, cytoplasmic(Homo sapiens (Human))
Australian National University
Curated by ChEMBL
Australian National University
Curated by ChEMBL
Affinity DataIC50: 2.30E+3nMAssay Description:Compound was evaluated for its inhibitory activity against Leucyl-tRNA synthetase from staphylococcus aureus WCUH29More data for this Ligand-Target Pair
Affinity DataIC50: 3.27E+3nMAssay Description:Compound was evaluated for its inhibitory activity against Isoleucyl-tRNA synthetase from staphylococcus aureus WCUH29More data for this Ligand-Target Pair
Affinity DataIC50: 6.10E+3nMAssay Description:Compound was evaluated for its inhibitory activity against Isoleucyl-tRNA synthetase from rat liverMore data for this Ligand-Target Pair
Affinity DataIC50: 1.46E+4nMAssay Description:Compound was evaluated for its inhibitory activity against Isoleucyl-tRNA synthetase from staphylococcus aureus WCUH29More data for this Ligand-Target Pair
Affinity DataIC50: 2.23E+4nMAssay Description:Compound was evaluated for its inhibitory activity against Isoleucyl-tRNA synthetase from rat liverMore data for this Ligand-Target Pair
TargetLeucine--tRNA ligase, cytoplasmic(Homo sapiens (Human))
Australian National University
Curated by ChEMBL
Australian National University
Curated by ChEMBL
Affinity DataIC50: 1.86E+5nMAssay Description:Compound was evaluated for its inhibitory activity against Leucyl-tRNA synthetase from staphylococcus aureus WCUH29More data for this Ligand-Target Pair
Affinity DataIC50: 4.00E+7nMAssay Description:Compound was tested for inhibitory activity against fucosyltransferaseMore data for this Ligand-Target Pair
Affinity DataIC50: >1.25E+8nMAssay Description:Compound was tested for inhibitory activity against fucosyltransferaseMore data for this Ligand-Target Pair
Affinity DataIC50: >1.25E+8nMAssay Description:Compound was tested for inhibitory activity against fucosyltransferaseMore data for this Ligand-Target Pair
TargetMonocarboxylate transporter 1(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataEC50: 1.00E+3nMAssay Description:Inhibition of MCT1 in human Raji cells assessed as inhibition of cell proliferation after 96 hrs by MTT assayMore data for this Ligand-Target Pair
TargetMonocarboxylate transporter 1(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataEC50: 55nMAssay Description:Inhibition of MCT1 in human Raji cells assessed as inhibition of cell proliferation after 96 hrs by MTT assayMore data for this Ligand-Target Pair
TargetMonocarboxylate transporter 1(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataEC50: 60nMAssay Description:Inhibition of MCT1 in human Raji cells assessed as inhibition of cell proliferation after 96 hrs by MTT assayMore data for this Ligand-Target Pair
TargetMonocarboxylate transporter 1(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataEC50: 520nMAssay Description:Inhibition of MCT1 in human Raji cells assessed as inhibition of cell proliferation after 96 hrs by MTT assayMore data for this Ligand-Target Pair
TargetMonocarboxylate transporter 1(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataEC50: 24nMAssay Description:Inhibition of MCT1 in human Raji cells assessed as inhibition of cell proliferation after 96 hrs by MTT assayMore data for this Ligand-Target Pair
TargetMonocarboxylate transporter 1(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataEC50: 420nMAssay Description:Inhibition of MCT1 in human Raji cells assessed as inhibition of cell proliferation after 96 hrs by MTT assayMore data for this Ligand-Target Pair
TargetMonocarboxylate transporter 1(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataEC50: 0.800nMAssay Description:Inhibition of MCT1 in human Raji cells assessed as inhibition of cell proliferation after 96 hrs by MTT assayMore data for this Ligand-Target Pair
TargetMonocarboxylate transporter 1(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataEC50: 500nMAssay Description:Inhibition of MCT1 in human Raji cells assessed as inhibition of cell proliferation after 96 hrs by MTT assayMore data for this Ligand-Target Pair
TargetMonocarboxylate transporter 1(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataEC50: 0.800nMAssay Description:Inhibition of MCT1 in human Raji cells assessed as inhibition of cell proliferation after 96 hrs by MTT assayMore data for this Ligand-Target Pair
TargetMonocarboxylate transporter 1(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataEC50: 100nMAssay Description:Inhibition of MCT1 in human Raji cells assessed as inhibition of cell proliferation after 96 hrs by MTT assayMore data for this Ligand-Target Pair
TargetMonocarboxylate transporter 1(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataEC50: 0.160nMAssay Description:Inhibition of MCT1 in human Raji cells assessed as inhibition of cell proliferation after 96 hrs by MTT assayMore data for this Ligand-Target Pair
TargetMonocarboxylate transporter 1(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataEC50: 0.800nMAssay Description:Inhibition of MCT1 in human Raji cells assessed as inhibition of cell proliferation after 96 hrs by MTT assayMore data for this Ligand-Target Pair
TargetMonocarboxylate transporter 1(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataEC50: 4nMAssay Description:Inhibition of MCT1 in human Raji cells assessed as inhibition of cell proliferation after 96 hrs by MTT assayMore data for this Ligand-Target Pair
TargetMonocarboxylate transporter 1(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataEC50: 11nMAssay Description:Inhibition of MCT1 in human Raji cells assessed as inhibition of cell proliferation after 96 hrs by MTT assayMore data for this Ligand-Target Pair
TargetMonocarboxylate transporter 1(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataEC50: 5nMAssay Description:Inhibition of MCT1 in human Raji cells assessed as inhibition of cell proliferation after 96 hrs by MTT assayMore data for this Ligand-Target Pair
TargetMonocarboxylate transporter 1(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataEC50: 690nMAssay Description:Inhibition of MCT1 in human Raji cells assessed as inhibition of cell proliferation after 96 hrs by MTT assayMore data for this Ligand-Target Pair
TargetMalignant T-cell-amplified sequence 1(Homo sapiens (human))
The Scripps Research Institute
US Patent
The Scripps Research Institute
US Patent
Affinity DataEC50: <20nMAssay Description:pecific Examples 1-52 of compounds of the invention, with estimated EC50 values determined using an MTT assay for 4-day viability of Raji (Burkitt...More data for this Ligand-Target Pair
TargetMalignant T-cell-amplified sequence 1(Homo sapiens (human))
The Scripps Research Institute
US Patent
The Scripps Research Institute
US Patent
Affinity DataEC50: 100nMAssay Description:pecific Examples 1-52 of compounds of the invention, with estimated EC50 values determined using an MTT assay for 4-day viability of Raji (Burkitt...More data for this Ligand-Target Pair
TargetMalignant T-cell-amplified sequence 1(Homo sapiens (human))
The Scripps Research Institute
US Patent
The Scripps Research Institute
US Patent
Affinity DataEC50: 5.50E+3nMAssay Description:pecific Examples 1-52 of compounds of the invention, with estimated EC50 values determined using an MTT assay for 4-day viability of Raji (Burkitt...More data for this Ligand-Target Pair
TargetMalignant T-cell-amplified sequence 1(Homo sapiens (human))
The Scripps Research Institute
US Patent
The Scripps Research Institute
US Patent
Affinity DataEC50: 200nMAssay Description:pecific Examples 1-52 of compounds of the invention, with estimated EC50 values determined using an MTT assay for 4-day viability of Raji (Burkitt...More data for this Ligand-Target Pair
TargetMalignant T-cell-amplified sequence 1(Homo sapiens (human))
The Scripps Research Institute
US Patent
The Scripps Research Institute
US Patent
Affinity DataEC50: 160nMAssay Description:pecific Examples 1-52 of compounds of the invention, with estimated EC50 values determined using an MTT assay for 4-day viability of Raji (Burkitt...More data for this Ligand-Target Pair
TargetMalignant T-cell-amplified sequence 1(Homo sapiens (human))
The Scripps Research Institute
US Patent
The Scripps Research Institute
US Patent
Affinity DataEC50: <20nMAssay Description:pecific Examples 1-52 of compounds of the invention, with estimated EC50 values determined using an MTT assay for 4-day viability of Raji (Burkitt...More data for this Ligand-Target Pair
TargetMalignant T-cell-amplified sequence 1(Homo sapiens (human))
The Scripps Research Institute
US Patent
The Scripps Research Institute
US Patent
Affinity DataEC50: <20nMAssay Description:pecific Examples 1-52 of compounds of the invention, with estimated EC50 values determined using an MTT assay for 4-day viability of Raji (Burkitt...More data for this Ligand-Target Pair
TargetMalignant T-cell-amplified sequence 1(Homo sapiens (human))
The Scripps Research Institute
US Patent
The Scripps Research Institute
US Patent
Affinity DataEC50: 34nMAssay Description:pecific Examples 1-52 of compounds of the invention, with estimated EC50 values determined using an MTT assay for 4-day viability of Raji (Burkitt...More data for this Ligand-Target Pair
TargetMalignant T-cell-amplified sequence 1(Homo sapiens (human))
The Scripps Research Institute
US Patent
The Scripps Research Institute
US Patent
Affinity DataEC50: <20nMAssay Description:pecific Examples 1-52 of compounds of the invention, with estimated EC50 values determined using an MTT assay for 4-day viability of Raji (Burkitt...More data for this Ligand-Target Pair