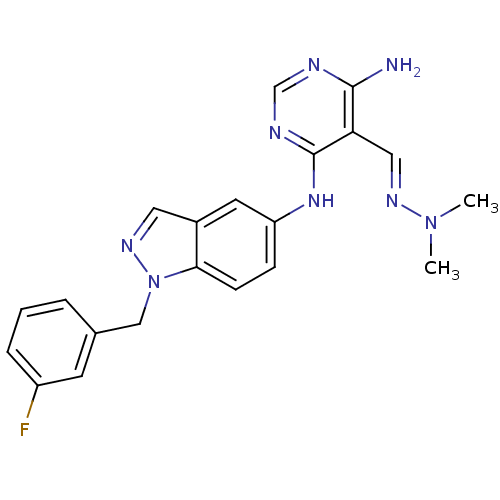
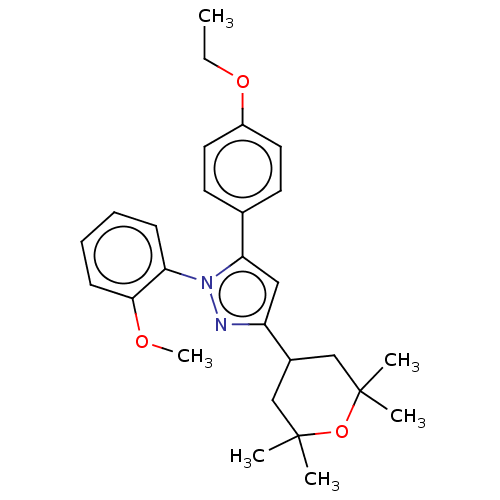
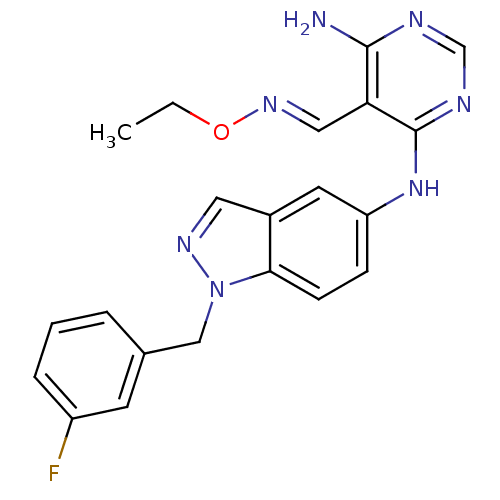
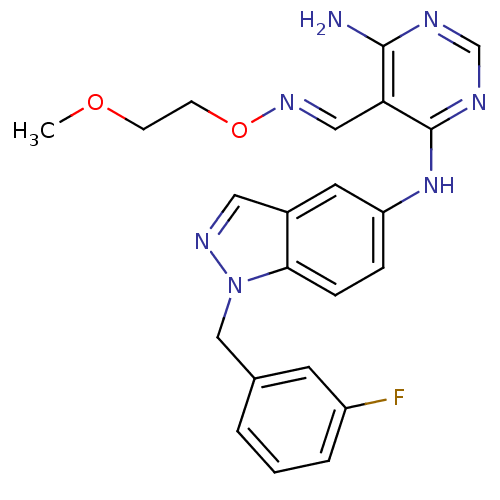
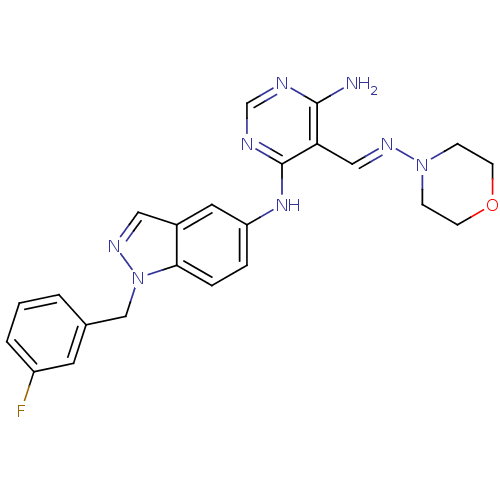
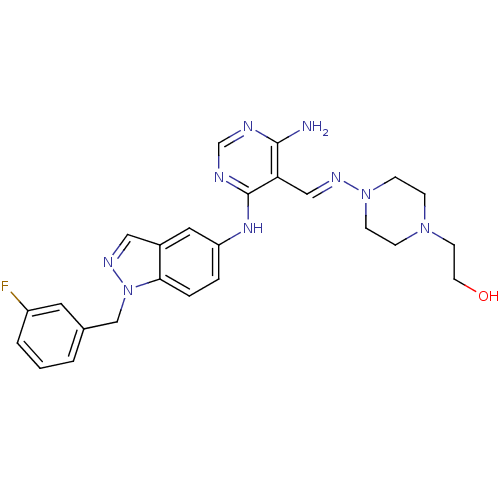
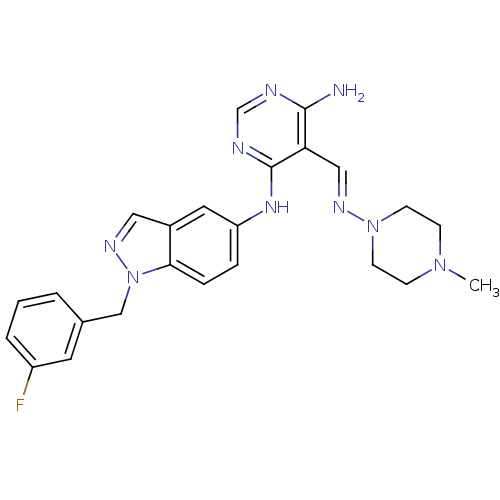
Affinity DataKi: 2.20nM ΔG°: -49.4kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: 3.10nM ΔG°: -48.6kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: 5.30nM ΔG°: -47.2kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: 13nM ΔG°: -45.0kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: 20nM ΔG°: -43.9kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: 26nM ΔG°: -43.3kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: 27nM ΔG°: -43.2kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: 34nM ΔG°: -42.6kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: 36nM ΔG°: -42.5kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: 63nM ΔG°: -41.1kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: 96nM ΔG°: -40.1kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: 160nM ΔG°: -38.8kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: 166nM ΔG°: -38.7kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: 170nM ΔG°: -38.6kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: 310nM ΔG°: -37.1kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: 355nM ΔG°: -36.8kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: 360nM ΔG°: -36.8kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: 470nM ΔG°: -36.1kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: 980nM ΔG°: -34.3kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: 1.16E+3nM ΔG°: -33.9kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: 8.90E+3nM ΔG°: -28.8kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: >1.28E+4nM ΔG°: >-27.9kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: >1.28E+4nM ΔG°: >-27.9kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: >1.28E+4nM ΔG°: >-27.9kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: >1.28E+4nM ΔG°: >-27.9kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: >1.28E+4nM ΔG°: >-27.9kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
TargetVoltage-dependent N-type calcium channel subunit alpha-1B(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of Cav2.2 (unknown origin) expressed in HEK293 cells assessed as decrease in KCl depolarization-induced Ca2+ influx measured for 5 mins by...More data for this Ligand-Target Pair
TargetVoltage-dependent N-type calcium channel subunit alpha-1B(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of Cav2.2 (unknown origin) expressed in HEK293 cells assessed as decrease in KCl depolarization-induced Ca2+ influx measured for 5 mins by...More data for this Ligand-Target Pair
TargetVoltage-dependent N-type calcium channel subunit alpha-1B(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of Cav2.2 (unknown origin) expressed in HEK293 cells assessed as decrease in KCl depolarization-induced Ca2+ influx measured for 5 mins by...More data for this Ligand-Target Pair
TargetVoltage-dependent N-type calcium channel subunit alpha-1B(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of Cav2.2 (unknown origin) expressed in HEK293 cells assessed as decrease in KCl depolarization-induced Ca2+ influx measured for 5 mins by...More data for this Ligand-Target Pair
TargetVoltage-dependent N-type calcium channel subunit alpha-1B(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of Cav2.2 (unknown origin) expressed in HEK293 cells assessed as decrease in KCl depolarization-induced Ca2+ influx measured for 5 mins by...More data for this Ligand-Target Pair
TargetReceptor tyrosine-protein kinase erbB-2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of human ErbB2More data for this Ligand-Target Pair
TargetVoltage-dependent N-type calcium channel subunit alpha-1B(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of Cav2.2 (unknown origin) expressed in HEK293 cells assessed as decrease in KCl depolarization-induced Ca2+ influx measured for 5 mins by...More data for this Ligand-Target Pair
TargetVoltage-dependent N-type calcium channel subunit alpha-1B(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of Cav2.2 (unknown origin) expressed in HEK293 cells assessed as decrease in KCl depolarization-induced Ca2+ influx measured for 5 mins by...More data for this Ligand-Target Pair
TargetVoltage-dependent N-type calcium channel subunit alpha-1B(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of Cav2.2 (unknown origin) expressed in HEK293 cells assessed as decrease in KCl depolarization-induced Ca2+ influx measured for 5 mins by...More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
TargetVoltage-dependent N-type calcium channel subunit alpha-1B(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of Cav2.2 (unknown origin) expressed in HEK293 cells assessed as decrease in KCl depolarization-induced Ca2+ influx measured for 5 mins by...More data for this Ligand-Target Pair
TargetVoltage-dependent N-type calcium channel subunit alpha-1B(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Inhibition of Cav2.2 (unknown origin) expressed in HEK293 cells assessed as decrease in KCl depolarization-induced Ca2+ influx measured for 5 mins by...More data for this Ligand-Target Pair
TargetVoltage-dependent N-type calcium channel subunit alpha-1B(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Inhibition of Cav2.2 (unknown origin) expressed in HEK293 cells assessed as decrease in KCl depolarization-induced Ca2+ influx measured for 5 mins by...More data for this Ligand-Target Pair
TargetReceptor tyrosine-protein kinase erbB-2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
TargetVoltage-dependent N-type calcium channel subunit alpha-1B(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataIC50: 7nMAssay Description:Inhibition of Cav2.2 (unknown origin) expressed in HEK293 cells assessed as decrease in KCl depolarization-induced Ca2+ influx measured for 5 mins by...More data for this Ligand-Target Pair
TargetReceptor tyrosine-protein kinase erbB-2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 7nMAssay Description:Inhibition of human ErbB2More data for this Ligand-Target Pair
TargetReceptor tyrosine-protein kinase erbB-2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
TargetVoltage-dependent N-type calcium channel subunit alpha-1B(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataIC50: 7nMAssay Description:Inhibition of Cav2.2 (unknown origin) expressed in HEK293 cells assessed as decrease in KCl depolarization-induced Ca2+ influx measured for 5 mins by...More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 8nMAssay Description:Inhibition of human EGFR expressed in SF9 cellsMore data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 8nMAssay Description:Inhibition of human EGFR expressed in SF9 cellsMore data for this Ligand-Target Pair
TargetReceptor tyrosine-protein kinase erbB-2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 8nMAssay Description:Inhibition of human ErbB2More data for this Ligand-Target Pair
TargetReceptor tyrosine-protein kinase erbB-2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 8nMAssay Description:Inhibition of human ErbB2More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 9nMAssay Description:Inhibition of human EGFR expressed in SF9 cellsMore data for this Ligand-Target Pair

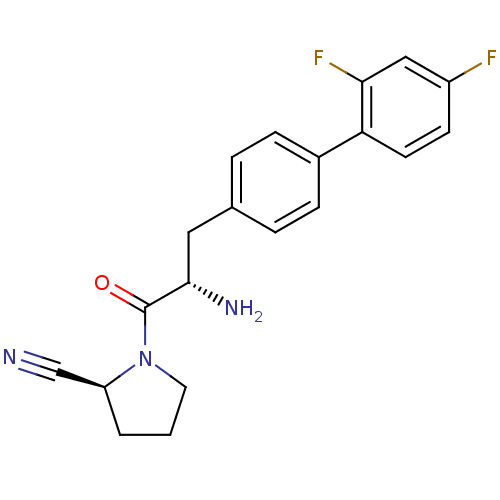





















 3D Structure (crystal)
3D Structure (crystal)