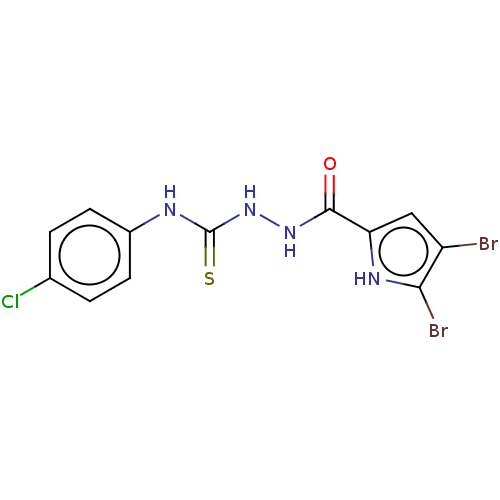
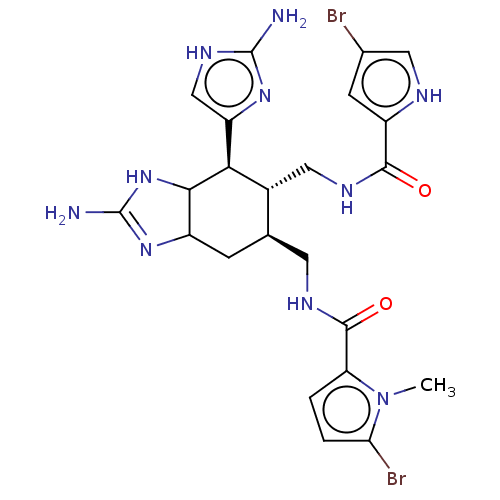
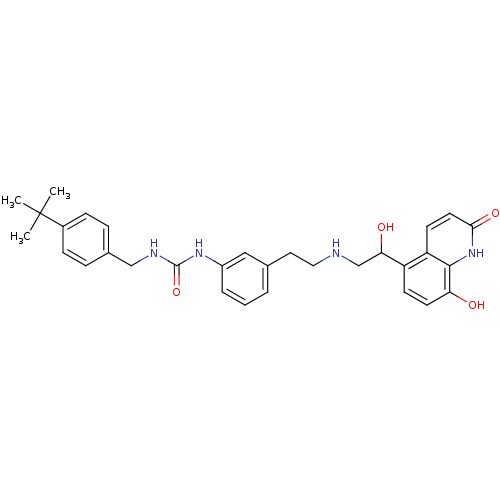
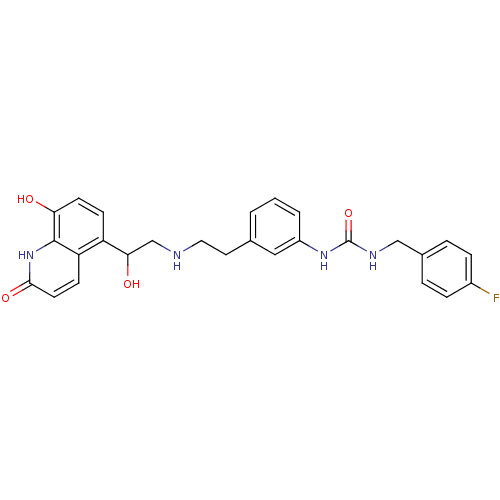
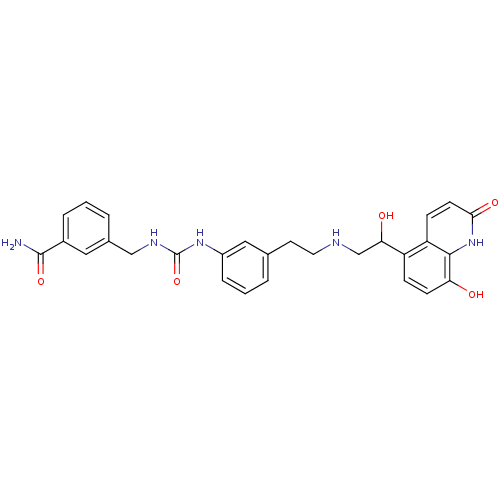
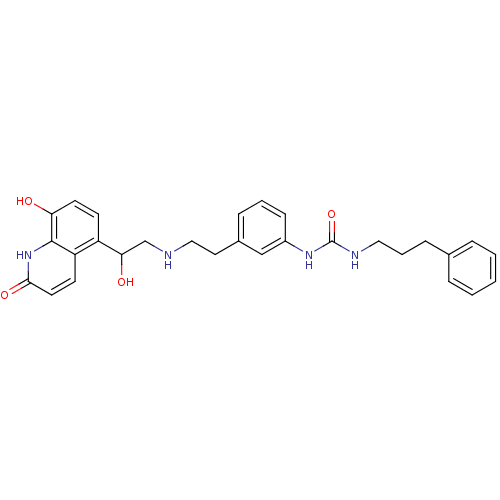
Affinity DataIC50: 20nMAssay Description:Inhibition of CDK4 (unknown origin)More data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
Ligand Info
Affinity DataIC50: 70nMAssay Description:Inhibition of GSK3beta (unknown origin)More data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
Ligand Info
Affinity DataIC50: 160nMAssay Description:Inhibition of CDK5/p25 (unknown origin)More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 1.80E+3nMAssay Description:Inhibition of MMP-2 (unknown origin) using succinylated gelatin as substrate incubated for 80 mins by microplate readerMore data for this Ligand-Target Pair
Affinity DataIC50: 1.80E+4nMAssay Description:Inhibition of Cbl-b (unknown origin) assessed as inhibition of Cbl-b dependent ubiquitination in presence of ATP measured after 13 hrs by fluorescenc...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 1.80E+4nMAssay Description:Inhibition of Cbl-b (unknown origin) assessed as inhibition of Cbl-b dependent ubiquitination in presence of ATP measured after 13 hrs by fluorescenc...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 1.80E+4nMAssay Description:Inhibition of Cbl-b (unknown origin) assessed as inhibition of Cbl-b dependent ubiquitination in presence of ATP measured after 13 hrs by fluorescenc...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 1.80E+4nMAssay Description:Inhibition of Cbl-b (unknown origin) assessed as inhibition of Cbl-b dependent ubiquitination in presence of ATP measured after 13 hrs by fluorescenc...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 1.80E+4nMAssay Description:Inhibition of Cbl-b (unknown origin) assessed as inhibition of Cbl-b dependent ubiquitination in presence of ATP measured after 13 hrs by fluorescenc...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 1.80E+4nMAssay Description:Inhibition of Cbl-b (unknown origin) assessed as inhibition of Cbl-b dependent ubiquitination in presence of ATP measured after 13 hrs by fluorescenc...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 1.80E+4nMAssay Description:Inhibition of Cbl-b (unknown origin) assessed as inhibition of Cbl-b dependent ubiquitination in presence of ATP measured after 13 hrs by fluorescenc...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 1.85E+4nMAssay Description:Inhibition of c-ErbB-2 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.92E+4nMAssay Description:Displacement of [125I]-VIP from Vasoactive intestinal peptide (unknown origin) incubated for 120 mins by gamma scintillation counter analysisMore data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 1.98E+4nMAssay Description:Displacement of [125I]-VIP from Vasoactive intestinal peptide (unknown origin) incubated for 120 mins by gamma scintillation counter analysisMore data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 2.10E+4nMAssay Description:Inhibition of c-ErbB-2 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 2.96E+4nMAssay Description:Inhibition of CDK4 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.79E+4nMAssay Description:Inhibition of c-ErbB-2 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.79E+4nMAssay Description:Inhibition of c-ErbB-2 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 4.46E+4nMAssay Description:Inhibition of EGFR (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 4.46E+4nMAssay Description:Inhibition of EGFR (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 9.85E+4nMAssay Description:Inhibition of CDK4 (unknown origin)More data for this Ligand-Target Pair
Affinity DataEC50: 1nMAssay Description:Agonist activity at beta2 adrenoreceptor in guinea pig tracheal rings assessed as vasorelaxationMore data for this Ligand-Target Pair
Affinity DataEC50: >1.00E+4nMAssay Description:Agonist activity at beta1 adrenoreceptor in rat left atria assessed as induction of ionotropic effectMore data for this Ligand-Target Pair
Affinity DataEC50: 900nMAssay Description:Agonist activity at beta1 adrenoreceptor in rat left atria assessed as induction of ionotropic effectMore data for this Ligand-Target Pair
Affinity DataEC50: >1.00E+4nMAssay Description:Agonist activity at beta1 adrenoreceptor in rat left atria assessed as induction of ionotropic effectMore data for this Ligand-Target Pair
Affinity DataEC50: 0.900nMAssay Description:Agonist activity at beta2 adrenoreceptor in guinea pig tracheal rings assessed as vasorelaxationMore data for this Ligand-Target Pair
Affinity DataEC50: 1nMAssay Description:Agonist activity at beta2 adrenoreceptor in guinea pig tracheal rings assessed as vasorelaxationMore data for this Ligand-Target Pair
Affinity DataEC50: 3.20nMAssay Description:Agonist activity at beta2 adrenoreceptor in guinea pig tracheal rings assessed as vasorelaxationMore data for this Ligand-Target Pair
Affinity DataEC50: 0.700nMAssay Description:Agonist activity at beta2 adrenoreceptor in guinea pig tracheal rings assessed as vasorelaxationMore data for this Ligand-Target Pair
Affinity DataEC50: 0.200nMAssay Description:Agonist activity at beta2 adrenoreceptor in guinea pig tracheal rings assessed as vasorelaxationMore data for this Ligand-Target Pair
Affinity DataEC50: 0.200nMAssay Description:Agonist activity at beta2 adrenoreceptor in guinea pig tracheal rings assessed as vasorelaxationMore data for this Ligand-Target Pair
Affinity DataEC50: 0.200nMAssay Description:Agonist activity at beta2 adrenoreceptor in guinea pig tracheal rings assessed as vasorelaxationMore data for this Ligand-Target Pair
Affinity DataEC50: 0.200nMAssay Description:Agonist activity at beta2 adrenoreceptor in guinea pig tracheal rings assessed as vasorelaxationMore data for this Ligand-Target Pair
Affinity DataEC50: 0.130nMAssay Description:Agonist activity at beta2 adrenoreceptor in guinea pig tracheal rings assessed as vasorelaxationMore data for this Ligand-Target Pair
Affinity DataEC50: 0.0500nMAssay Description:Agonist activity at beta2 adrenoreceptor in guinea pig tracheal rings assessed as vasorelaxationMore data for this Ligand-Target Pair
Affinity DataEC50: 0.0900nMAssay Description:Agonist activity at beta2 adrenoreceptor in guinea pig tracheal rings assessed as vasorelaxationMore data for this Ligand-Target Pair
Affinity DataEC50: 0.200nMAssay Description:Agonist activity at beta2 adrenoreceptor in guinea pig tracheal rings assessed as vasorelaxationMore data for this Ligand-Target Pair
Affinity DataEC50: 0.0700nMAssay Description:Agonist activity at beta2 adrenoreceptor in guinea pig tracheal rings assessed as vasorelaxationMore data for this Ligand-Target Pair
Affinity DataEC50: 2.31E+3nMAssay Description:Agonist activity at beta1 adrenoreceptor in rat left atria assessed as induction of ionotropic effectMore data for this Ligand-Target Pair
Affinity DataEC50: 1.00E+4nMAssay Description:Agonist activity at beta1 adrenoreceptor in rat left atria assessed as induction of ionotropic effectMore data for this Ligand-Target Pair
Affinity DataEC50: 296nMAssay Description:Agonist activity at beta1 adrenoreceptor in rat left atria assessed as induction of ionotropic effectMore data for this Ligand-Target Pair
Affinity DataEC50: 5.77E+3nMAssay Description:Agonist activity at beta1 adrenoreceptor in rat left atria assessed as induction of ionotropic effectMore data for this Ligand-Target Pair
Affinity DataEC50: 8.89E+3nMAssay Description:Agonist activity at beta1 adrenoreceptor in rat left atria assessed as induction of ionotropic effectMore data for this Ligand-Target Pair
Affinity DataEC50: 1.60E+3nMAssay Description:Agonist activity at beta1 adrenoreceptor in rat left atria assessed as induction of ionotropic effectMore data for this Ligand-Target Pair
Affinity DataEC50: 6.30E+3nMAssay Description:Agonist activity at beta1 adrenoreceptor in rat left atria assessed as induction of ionotropic effectMore data for this Ligand-Target Pair
Affinity DataEC50: >1.00E+4nMAssay Description:Agonist activity at beta1 adrenoreceptor in rat left atria assessed as induction of ionotropic effectMore data for this Ligand-Target Pair
Affinity DataEC50: 550nMAssay Description:Agonist activity at beta1 adrenoreceptor in rat left atria assessed as induction of ionotropic effectMore data for this Ligand-Target Pair