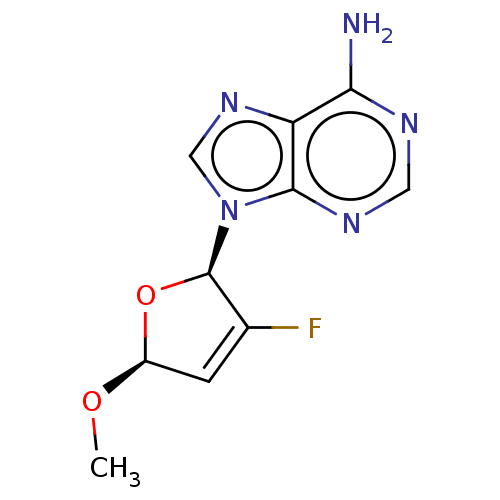
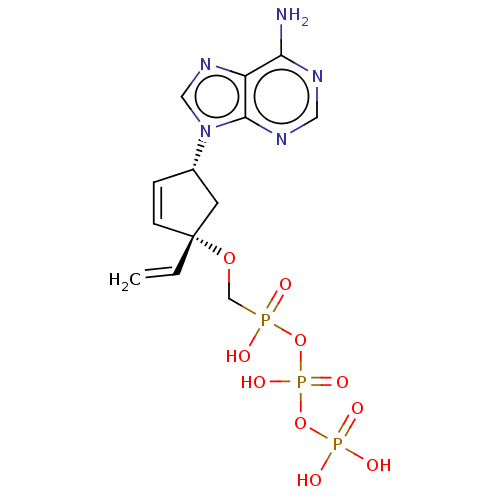
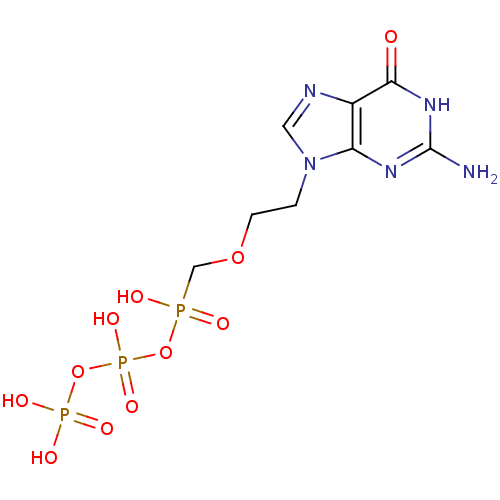
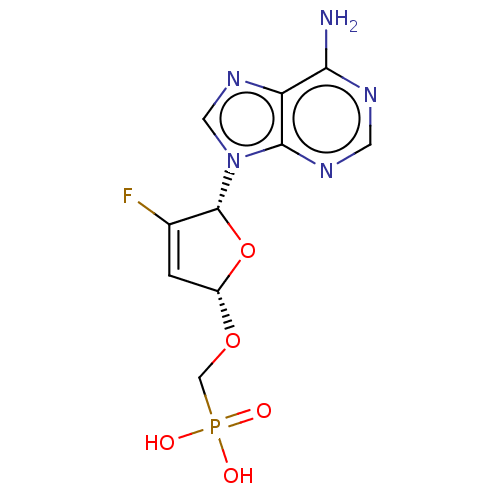
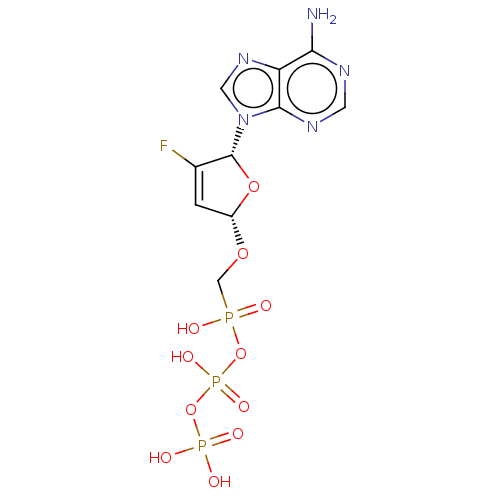
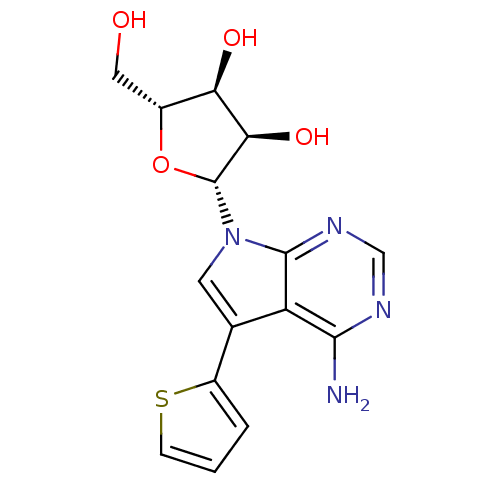
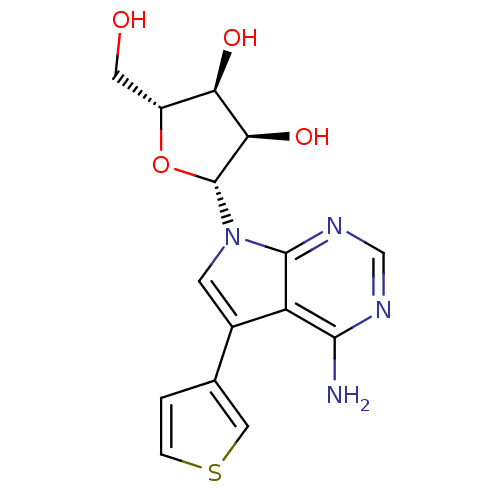
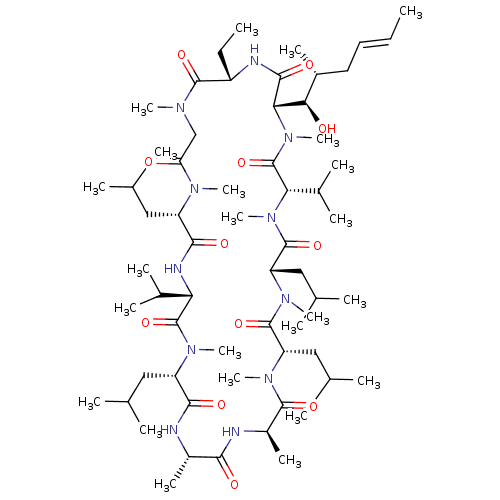
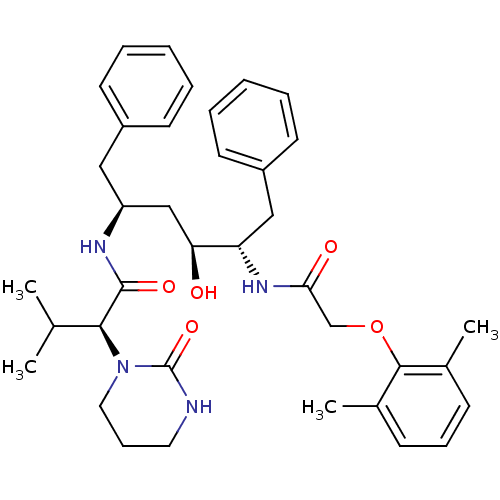
Affinity DataKi: 6.10E+4nMAssay Description:Uncompetitive inhibition of Trypanosoma cruzi trypanothione reductase using T(SH)2 as substrate at pH 7.5 in presence of NADPH by photometric methodMore data for this Ligand-Target Pair
Affinity DataKi: 6.80E+4nMAssay Description:Uncompetitive inhibition of Trypanosoma cruzi trypanothione reductase using T(SH)2 as substrate at pH 7.5 in presence of NADPH by photometric methodMore data for this Ligand-Target Pair
Affinity DataKi: 1.18E+5nMAssay Description:Uncompetitive inhibition of Trypanosoma cruzi trypanothione reductase using T(SH)2 as substrate at pH 7.5 in presence of NADPH by photometric methodMore data for this Ligand-Target Pair
Affinity DataKi: 1.28E+5nMAssay Description:Uncompetitive inhibition of Trypanosoma cruzi trypanothione reductase using T(SH)2 as substrate at pH 7.5 in presence of NADPH by photometric methodMore data for this Ligand-Target Pair
Affinity DataKi: 1.36E+5nMAssay Description:Uncompetitive inhibition of Trypanosoma cruzi trypanothione reductase using T(SH)2 as substrate at pH 7.5 in presence of NADPH by photometric methodMore data for this Ligand-Target Pair
Affinity DataKi: 1.83E+5nMAssay Description:Uncompetitive inhibition of Trypanosoma cruzi trypanothione reductase using T(SH)2 as substrate at pH 7.5 in presence of NADPH by photometric methodMore data for this Ligand-Target Pair
Affinity DataKi: 1.87E+5nMAssay Description:Uncompetitive inhibition of Trypanosoma cruzi trypanothione reductase using T(SH)2 as substrate at pH 7.5 in presence of NADPH by photometric methodMore data for this Ligand-Target Pair
Affinity DataKi: 2.45E+5nMAssay Description:Uncompetitive inhibition of Trypanosoma cruzi trypanothione reductase using T(SH)2 as substrate at pH 7.5 in presence of NADPH by photometric methodMore data for this Ligand-Target Pair
Affinity DataKi: 2.82E+5nMAssay Description:Uncompetitive inhibition of Trypanosoma cruzi trypanothione reductase using T(SH)2 as substrate at pH 7.5 in presence of NADPH by photometric methodMore data for this Ligand-Target Pair
Affinity DataKi: 3.13E+5nMAssay Description:Uncompetitive inhibition of Trypanosoma cruzi trypanothione reductase using T(SH)2 as substrate at pH 7.5 in presence of NADPH by photometric methodMore data for this Ligand-Target Pair
Affinity DataKi: 3.15E+5nMAssay Description:Uncompetitive inhibition of Trypanosoma cruzi trypanothione reductase using T(SH)2 as substrate at pH 7.5 in presence of NADPH by photometric methodMore data for this Ligand-Target Pair
Affinity DataKi: 3.36E+5nMAssay Description:Uncompetitive inhibition of Trypanosoma cruzi trypanothione reductase using T(SH)2 as substrate at pH 7.5 in presence of NADPH by photometric methodMore data for this Ligand-Target Pair
Affinity DataKi: 4.14E+5nMAssay Description:Uncompetitive inhibition of Trypanosoma cruzi trypanothione reductase using T(SH)2 as substrate at pH 7.5 in presence of NADPH by photometric methodMore data for this Ligand-Target Pair
Affinity DataKi: 1.18E+6nMAssay Description:Uncompetitive inhibition of Trypanosoma cruzi trypanothione reductase using T(SH)2 as substrate at pH 7.5 in presence of NADPH by photometric methodMore data for this Ligand-Target Pair
Affinity DataIC50: 50nMAssay Description:Inhibition of wild type HIV 3B reverse transcriptase in MT2 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 50nMAssay Description:Inhibition on HIV1 reverse transcriptase p66/p51More data for this Ligand-Target Pair
TargetAdenosine kinase(Homo sapiens (Human))
Academy Of Sciences Of The Czech Republic
Curated by ChEMBL
Academy Of Sciences Of The Czech Republic
Curated by ChEMBL
Affinity DataIC50: 50nMAssay Description:Inhibition of human ADK using [3H]-adenosine by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 130nMAssay Description:Inhibition on HIV1 reverse transcriptase p66/p51More data for this Ligand-Target Pair
Affinity DataIC50: 130nMAssay Description:Inhibition on HIV1 reverse transcriptase p66/p51More data for this Ligand-Target Pair
TargetAdenosine kinase(Homo sapiens (Human))
Academy Of Sciences Of The Czech Republic
Curated by ChEMBL
Academy Of Sciences Of The Czech Republic
Curated by ChEMBL
Affinity DataIC50: 200nMAssay Description:Inhibition of human ADK using [3H]-adenosine by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 380nMAssay Description:Inhibition of wild type HIV 3B reverse transcriptase in MT2 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 380nMAssay Description:Inhibition on HIV1 reverse transcriptase p66/p51More data for this Ligand-Target Pair
Affinity DataIC50: 400nMAssay Description:Inhibition of wild type HIV 3B reverse transcriptase in MT2 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 600nMAssay Description:Inhibition on HIV1 reverse transcriptase p66/p51More data for this Ligand-Target Pair
Affinity DataIC50: 600nMAssay Description:Inhibition of wild type HIV 3B reverse transcriptase in MT2 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 620nMAssay Description:Inhibition on HIV1 reverse transcriptase p66/p51More data for this Ligand-Target Pair
Affinity DataIC50: 1.40E+3nMAssay Description:Inhibition of human DNA polymerase-betaMore data for this Ligand-Target Pair
Affinity DataIC50: 1.60E+3nMAssay Description:Inhibition of human DNA polymerase beta by microplate reader analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.90E+3nMAssay Description:Inhibition on HIV1 reverse transcriptase p66/p51More data for this Ligand-Target Pair
Affinity DataIC50: 1.90E+3nMAssay Description:Inhibition of HIV1 reverse transcriptaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.90E+3nMAssay Description:Inhibition of wild type HIV 3B reverse transcriptase in MT2 cellsMore data for this Ligand-Target Pair
TargetAdenosine kinase(Homo sapiens (Human))
Academy Of Sciences Of The Czech Republic
Curated by ChEMBL
Academy Of Sciences Of The Czech Republic
Curated by ChEMBL
Affinity DataIC50: >2.00E+3nMAssay Description:Inhibition of human ADK using [3H]-adenosine by scintillation countingMore data for this Ligand-Target Pair
TargetAdenosine kinase(Homo sapiens (Human))
Academy Of Sciences Of The Czech Republic
Curated by ChEMBL
Academy Of Sciences Of The Czech Republic
Curated by ChEMBL
Affinity DataIC50: >2.00E+3nMAssay Description:Inhibition of human ADK using [3H]-adenosine by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+3nMAssay Description:Inhibition of human DNA polymerase alpha by microplate reader analysisMore data for this Ligand-Target Pair
TargetAdenosine kinase(Homo sapiens (Human))
Academy Of Sciences Of The Czech Republic
Curated by ChEMBL
Academy Of Sciences Of The Czech Republic
Curated by ChEMBL
Affinity DataIC50: >5.00E+3nMAssay Description:Inhibition of human ADK using [3H]-adenosine by scintillation countingMore data for this Ligand-Target Pair
TargetAdenosine kinase(Homo sapiens (Human))
Academy Of Sciences Of The Czech Republic
Curated by ChEMBL
Academy Of Sciences Of The Czech Republic
Curated by ChEMBL
Affinity DataIC50: >5.00E+3nMAssay Description:Inhibition of human ADK using [3H]-adenosine by scintillation countingMore data for this Ligand-Target Pair
TargetAdenosine kinase(Homo sapiens (Human))
Academy Of Sciences Of The Czech Republic
Curated by ChEMBL
Academy Of Sciences Of The Czech Republic
Curated by ChEMBL
Affinity DataIC50: >5.00E+3nMAssay Description:Inhibition of human ADK using [3H]-adenosine by scintillation countingMore data for this Ligand-Target Pair
TargetAdenosine kinase(Homo sapiens (Human))
Academy Of Sciences Of The Czech Republic
Curated by ChEMBL
Academy Of Sciences Of The Czech Republic
Curated by ChEMBL
Affinity DataIC50: >5.00E+3nMAssay Description:Inhibition of human ADK using [3H]-adenosine by scintillation countingMore data for this Ligand-Target Pair
TargetAdenosine kinase(Homo sapiens (Human))
Academy Of Sciences Of The Czech Republic
Curated by ChEMBL
Academy Of Sciences Of The Czech Republic
Curated by ChEMBL
Affinity DataIC50: >5.00E+3nMAssay Description:Inhibition of human ADK using [3H]-adenosine by scintillation countingMore data for this Ligand-Target Pair
TargetAdenosine kinase(Homo sapiens (Human))
Academy Of Sciences Of The Czech Republic
Curated by ChEMBL
Academy Of Sciences Of The Czech Republic
Curated by ChEMBL
Affinity DataIC50: >5.00E+3nMAssay Description:Inhibition of human ADK using [3H]-adenosine by scintillation countingMore data for this Ligand-Target Pair
TargetAdenosine kinase(Homo sapiens (Human))
Academy Of Sciences Of The Czech Republic
Curated by ChEMBL
Academy Of Sciences Of The Czech Republic
Curated by ChEMBL
Affinity DataIC50: >5.00E+3nMAssay Description:Inhibition of human ADK using [3H]-adenosine by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 9.34E+3nMAssay Description:Inhibition of human MDR1-dependent accumulation of calcein-AM expressed in MDCK2 cellsMore data for this Ligand-Target Pair
TargetAdenosine kinase(Homo sapiens (Human))
Academy Of Sciences Of The Czech Republic
Curated by ChEMBL
Academy Of Sciences Of The Czech Republic
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of human ADK using [3H]-adenosine by scintillation countingMore data for this Ligand-Target Pair
TargetAdenosine kinase(Homo sapiens (Human))
Academy Of Sciences Of The Czech Republic
Curated by ChEMBL
Academy Of Sciences Of The Czech Republic
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of human ADK using [3H]-adenosine by scintillation countingMore data for this Ligand-Target Pair
TargetAdenosine kinase(Homo sapiens (Human))
Academy Of Sciences Of The Czech Republic
Curated by ChEMBL
Academy Of Sciences Of The Czech Republic
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of human ADK using [3H]-adenosine by scintillation countingMore data for this Ligand-Target Pair
TargetAdenosine kinase(Homo sapiens (Human))
Academy Of Sciences Of The Czech Republic
Curated by ChEMBL
Academy Of Sciences Of The Czech Republic
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of human ADK using [3H]-adenosine by scintillation countingMore data for this Ligand-Target Pair
TargetAdenosine kinase(Homo sapiens (Human))
Academy Of Sciences Of The Czech Republic
Curated by ChEMBL
Academy Of Sciences Of The Czech Republic
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of human ADK using [3H]-adenosine by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 1.03E+4nMAssay Description:Inhibition of human MDR1-dependent accumulation of calcein-AM expressed in MDCK2 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.35E+4nMAssay Description:Inhibition of human DNA polymerase beta by microplate reader analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.99E+4nMAssay Description:Inhibition of human MDR1-dependent accumulation of calcein-AM expressed in MDCK2 cellsMore data for this Ligand-Target Pair



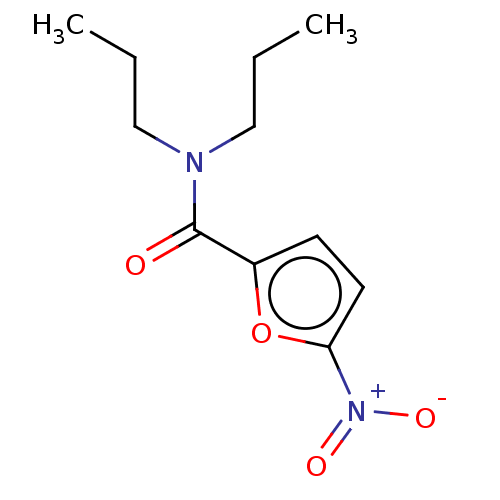


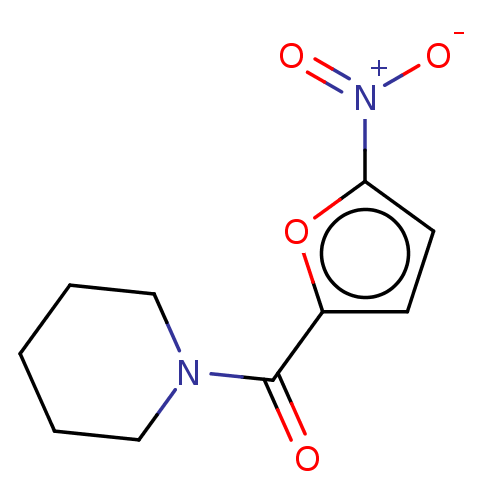
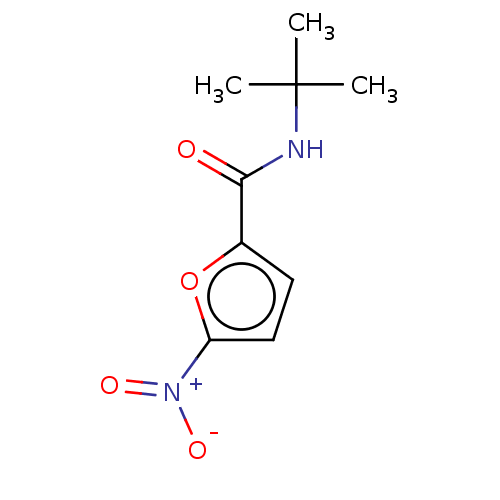


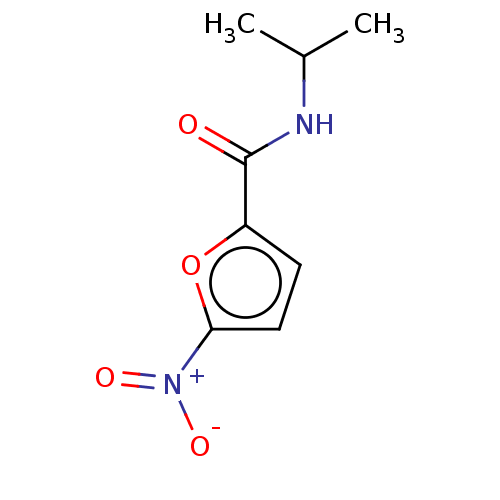





 3D Structure (crystal)
3D Structure (crystal)