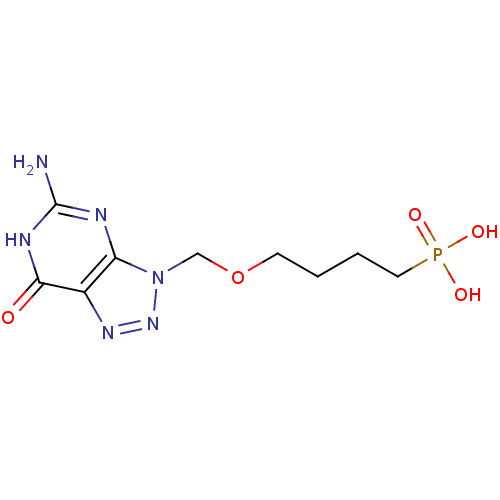
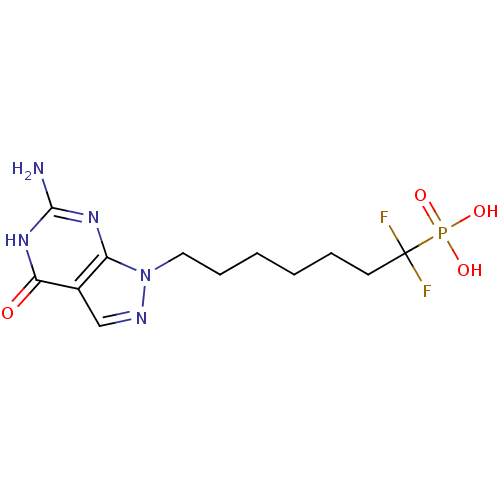
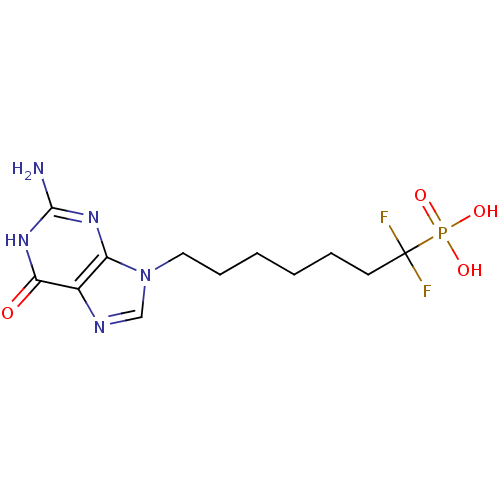
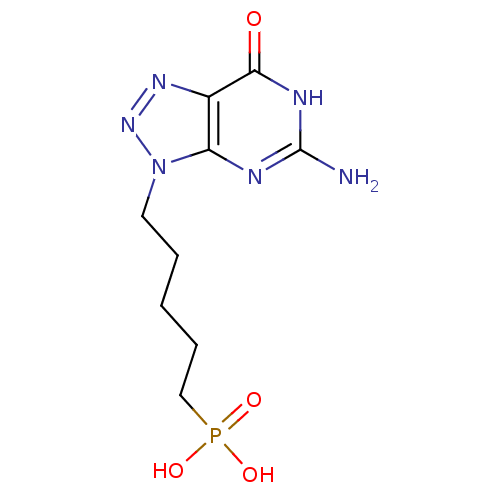
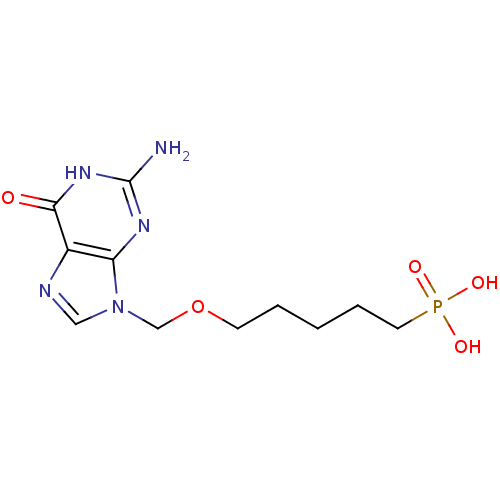
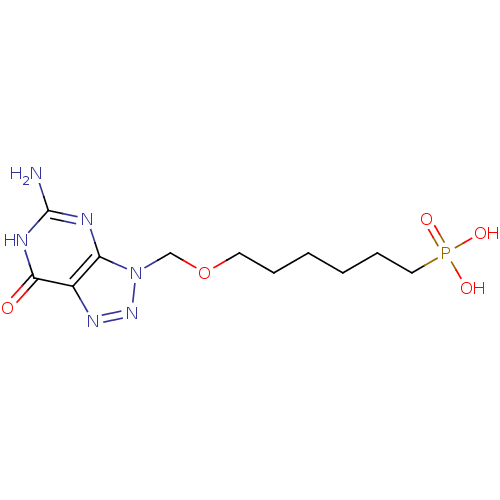
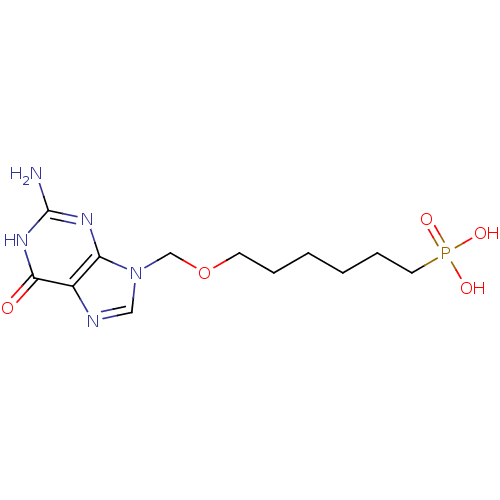
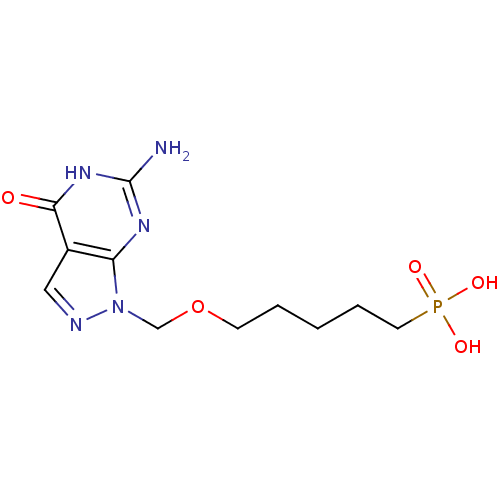
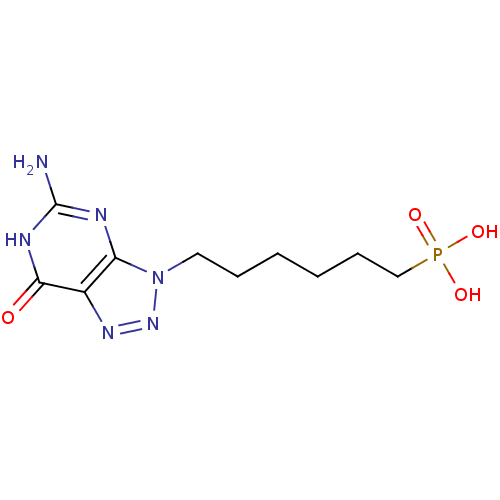
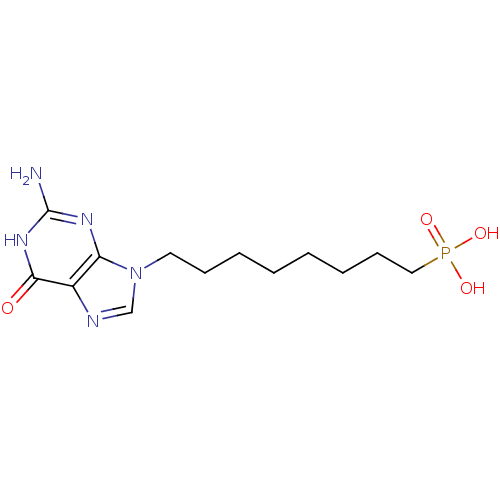
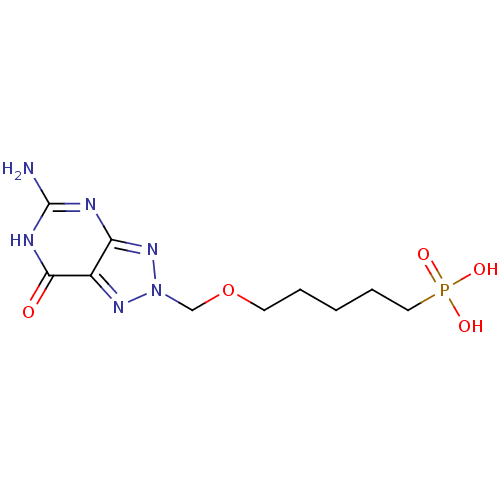
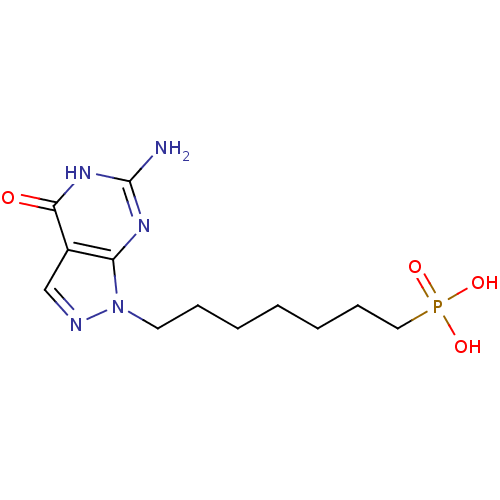
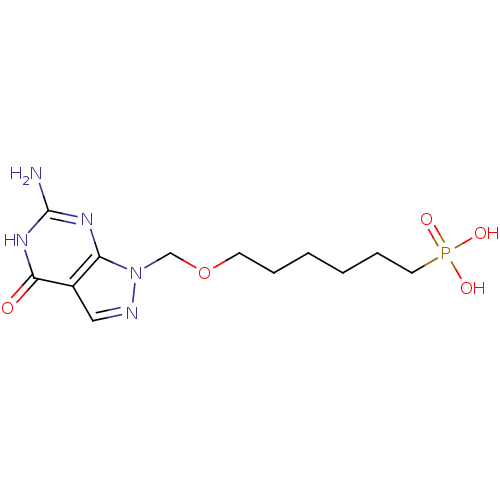
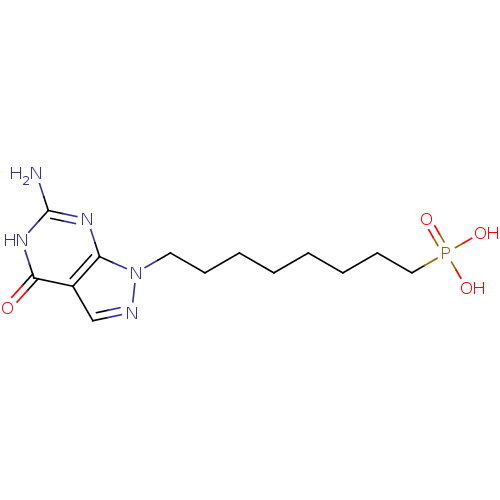
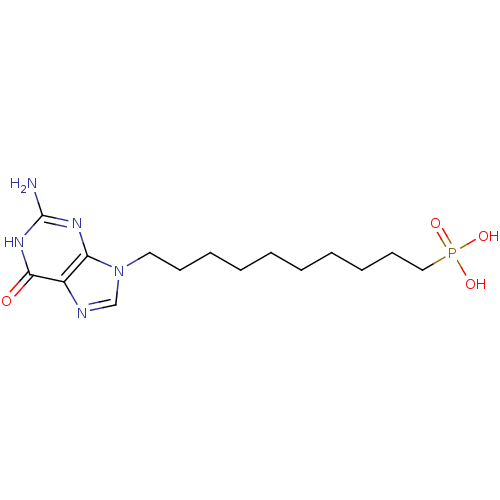
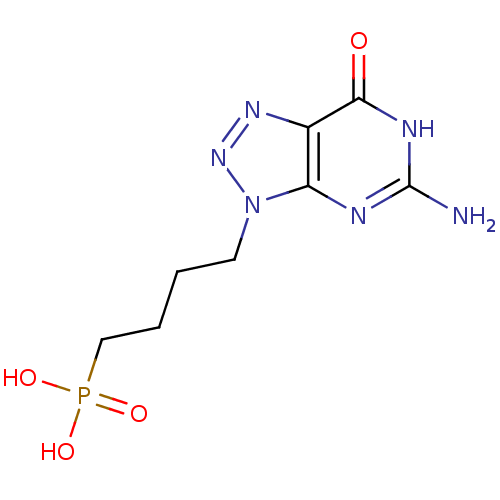
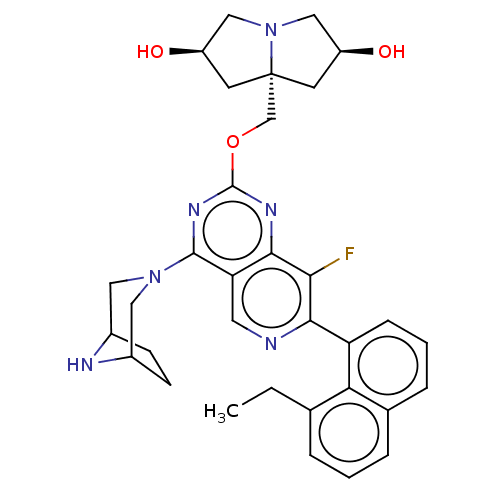
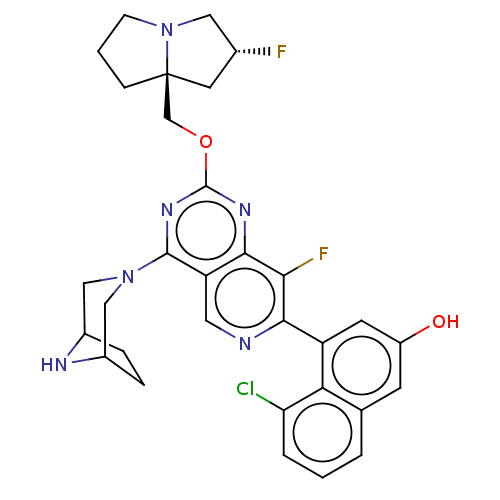
Affinity DataKi: 10nMAssay Description:In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes.More data for this Ligand-Target Pair
Affinity DataKi: 48nMAssay Description:In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes.More data for this Ligand-Target Pair
Affinity DataKi: 49nMAssay Description:In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes.More data for this Ligand-Target Pair
Affinity DataKi: 51nMAssay Description:In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes.More data for this Ligand-Target Pair
Affinity DataKi: 56nMAssay Description:In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes.More data for this Ligand-Target Pair
Affinity DataKi: 57nMAssay Description:In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes.More data for this Ligand-Target Pair
Affinity DataKi: 65nMAssay Description:In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes.More data for this Ligand-Target Pair
Affinity DataKi: 96nMAssay Description:In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes.More data for this Ligand-Target Pair
Affinity DataKi: 160nMAssay Description:In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes.More data for this Ligand-Target Pair
Affinity DataKi: 190nMAssay Description:In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes.More data for this Ligand-Target Pair
Affinity DataKi: 220nMAssay Description:In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes.More data for this Ligand-Target Pair
Affinity DataKi: 230nMAssay Description:In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes.More data for this Ligand-Target Pair
Affinity DataKi: 360nMAssay Description:In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes.More data for this Ligand-Target Pair
Affinity DataKi: 390nMAssay Description:In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes.More data for this Ligand-Target Pair
Affinity DataKi: 530nMAssay Description:In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes.More data for this Ligand-Target Pair
Affinity DataKi: 550nMAssay Description:In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes.More data for this Ligand-Target Pair
Affinity DataKi: 580nMAssay Description:In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes.More data for this Ligand-Target Pair
Affinity DataKi: 630nMAssay Description:In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes.More data for this Ligand-Target Pair
Affinity DataKi: 1.10E+3nMAssay Description:In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes.More data for this Ligand-Target Pair
Affinity DataKi: 1.60E+3nMAssay Description:In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes.More data for this Ligand-Target Pair
Affinity DataKi: 3.70E+3nMAssay Description:Inhibition of recombinant KRAS G12C mutant (unknown origin) assessed as rate of inactivation by LC-MS analysisMore data for this Ligand-Target Pair
Affinity DataKi: 7.30E+3nMAssay Description:In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes.More data for this Ligand-Target Pair
Affinity DataIC50: 0.00100nMAssay Description:This Example illustrates that exemplary compounds of the present invention inhibit the phosphorylation of ERK downstream of KRAS G12D. AGS cells (ATC...More data for this Ligand-Target Pair
Affinity DataIC50: <0.00200nMAssay Description:This Example illustrates that exemplary compounds of the present invention inhibit the phosphorylation of ERK downstream of KRAS G12D. AGS cells (ATC...More data for this Ligand-Target Pair
Affinity DataIC50: <0.00200nMAssay Description:This Example illustrates that exemplary compounds of the present invention inhibit the phosphorylation of ERK downstream of KRAS G12D. AGS cells (ATC...More data for this Ligand-Target Pair
Affinity DataIC50: <0.00200nMAssay Description:This Example illustrates that exemplary compounds of the present invention inhibit the phosphorylation of ERK downstream of KRAS G12D. AGS cells (ATC...More data for this Ligand-Target Pair
Affinity DataIC50: <0.00200nMAssay Description:This Example illustrates that exemplary compounds of the present invention inhibit the phosphorylation of ERK downstream of KRAS G12D. AGS cells (ATC...More data for this Ligand-Target Pair
Affinity DataIC50: <0.00200nMAssay Description:This Example illustrates that exemplary compounds of the present invention inhibit the phosphorylation of ERK downstream of KRAS G12D. AGS cells (ATC...More data for this Ligand-Target Pair
Affinity DataIC50: <0.00200nMAssay Description:This Example illustrates that exemplary compounds of the present invention inhibit the phosphorylation of ERK downstream of KRAS G12D. AGS cells (ATC...More data for this Ligand-Target Pair
Affinity DataIC50: 0.00600nMAssay Description:This Example illustrates that exemplary compounds of the present invention inhibit the phosphorylation of ERK downstream of KRAS G12D. AGS cells (ATC...More data for this Ligand-Target Pair
Affinity DataIC50: 0.00700nMAssay Description:This Example illustrates that exemplary compounds of the present invention inhibit the phosphorylation of ERK downstream of KRAS G12D. AGS cells (ATC...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0120nMAssay Description:This Example illustrates that exemplary compounds of the present invention inhibit the phosphorylation of ERK downstream of KRAS G12D. AGS cells (ATC...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0270nMAssay Description:This Example illustrates that exemplary compounds of the present invention inhibit the phosphorylation of ERK downstream of KRAS G12D. AGS cells (ATC...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0620nMAssay Description:This Example illustrates that exemplary compounds of the present invention inhibit the phosphorylation of ERK downstream of KRAS G12D. AGS cells (ATC...More data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMAssay Description:This Example illustrates that exemplary compounds of the present invention inhibit the phosphorylation of ERK downstream of KRAS G12D. AGS cells (ATC...More data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMAssay Description:Table 2: The ability of a compound to bind to KRAS G12D was measured using a TR-FRET displacement assay. Biotinylated GDP-loaded recombinant human KR...More data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:This Example illustrates that exemplary compounds of the present invention inhibit the phosphorylation of ERK downstream of KRAS G12D. AGS cells (ATC...More data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:Table 2: The ability of a compound to bind to KRAS G12D was measured using a TR-FRET displacement assay. Biotinylated GDP-loaded recombinant human KR...More data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMAssay Description:This Example illustrates that exemplary compounds of the present invention inhibit the phosphorylation of ERK downstream of KRAS G12D. AGS cells (ATC...More data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMAssay Description:Table 2: The ability of a compound to bind to KRAS G12D was measured using a TR-FRET displacement assay. Biotinylated GDP-loaded recombinant human KR...More data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMAssay Description:This Example illustrates that exemplary compounds of the present invention inhibit the phosphorylation of ERK downstream of KRAS G12D. AGS cells (ATC...More data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMAssay Description:Table 2: The ability of a compound to bind to KRAS G12D was measured using a TR-FRET displacement assay. Biotinylated GDP-loaded recombinant human KR...More data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMAssay Description:Table 2: The ability of a compound to bind to KRAS G12D was measured using a TR-FRET displacement assay. Biotinylated GDP-loaded recombinant human KR...More data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMAssay Description:This Example illustrates that exemplary compounds of the present invention inhibit the phosphorylation of ERK downstream of KRAS G12D. AGS cells (ATC...More data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMAssay Description:This Example illustrates that exemplary compounds of the present invention inhibit the phosphorylation of ERK downstream of KRAS G12D. AGS cells (ATC...More data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMAssay Description:Table 2: The ability of a compound to bind to KRAS G12D was measured using a TR-FRET displacement assay. Biotinylated GDP-loaded recombinant human KR...More data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMAssay Description:Table 2: The ability of a compound to bind to KRAS G12D was measured using a TR-FRET displacement assay. Biotinylated GDP-loaded recombinant human KR...More data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMAssay Description:This Example illustrates that exemplary compounds of the present invention inhibit the phosphorylation of ERK downstream of KRAS G12D. AGS cells (ATC...More data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMAssay Description:This Example illustrates that exemplary compounds of the present invention inhibit the phosphorylation of ERK downstream of KRAS G12D. AGS cells (ATC...More data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMAssay Description:Table 2: The ability of a compound to bind to KRAS G12D was measured using a TR-FRET displacement assay. Biotinylated GDP-loaded recombinant human KR...More data for this Ligand-Target Pair