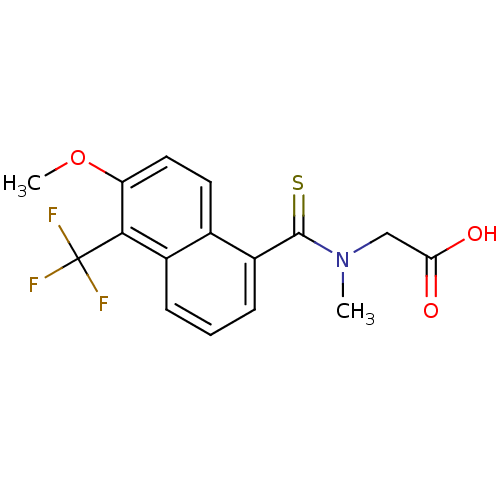
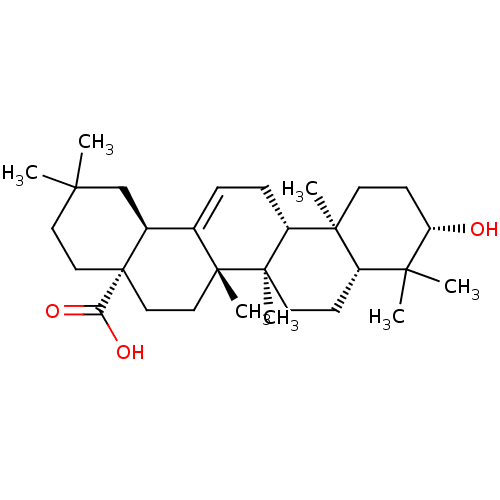
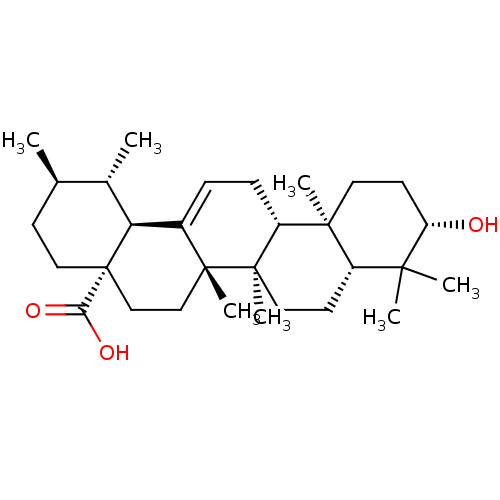
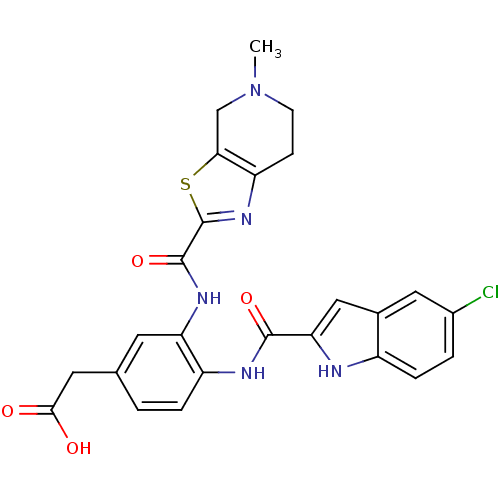
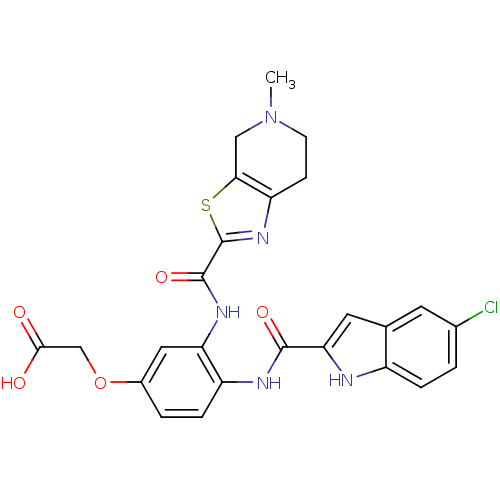
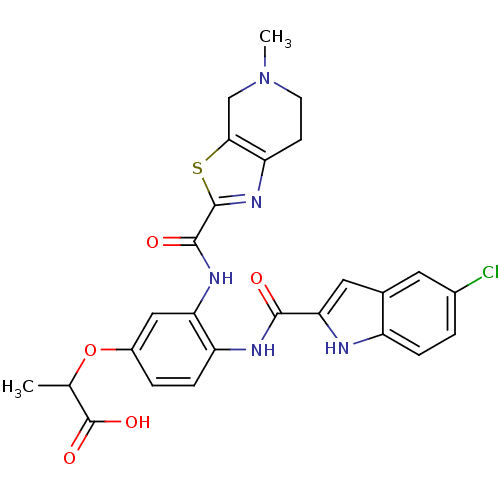
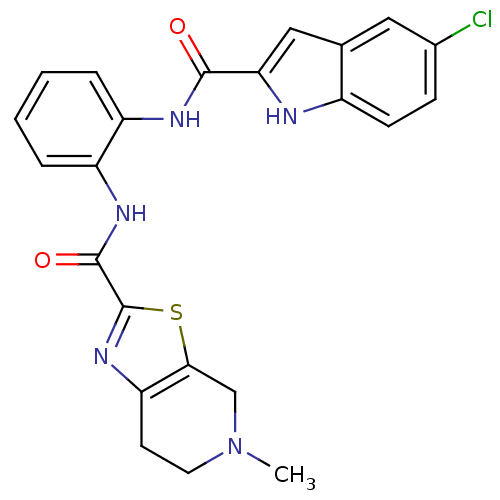
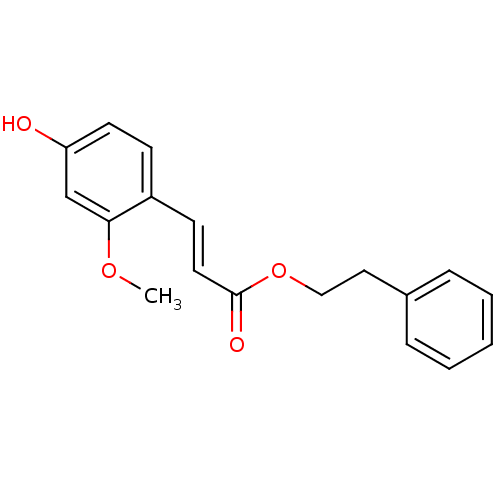
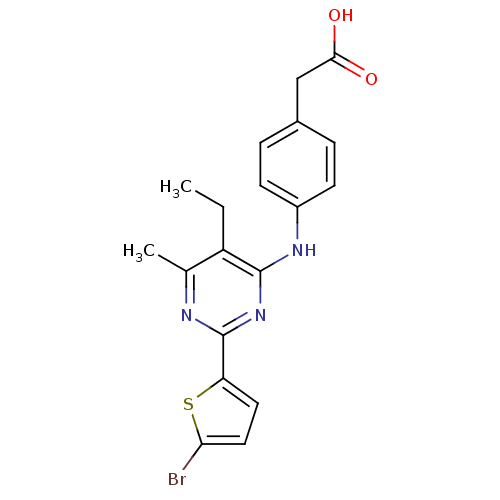
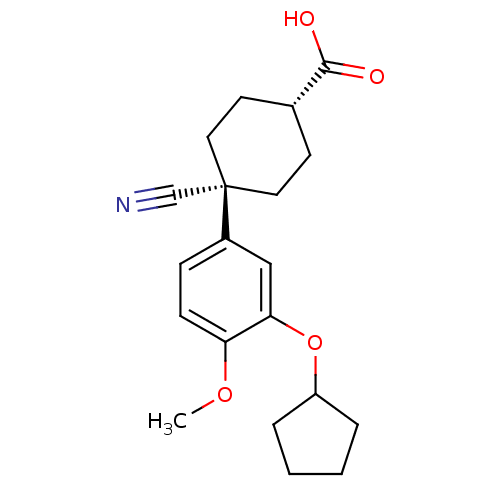
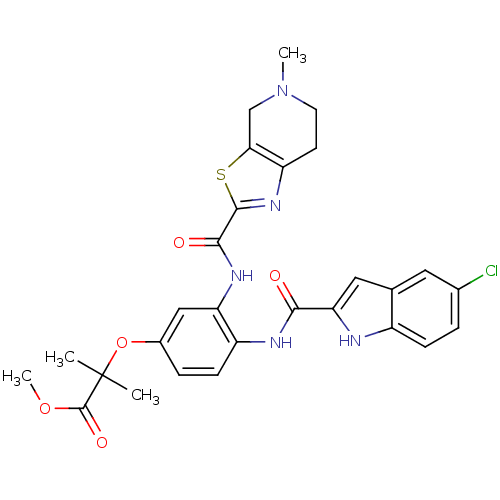
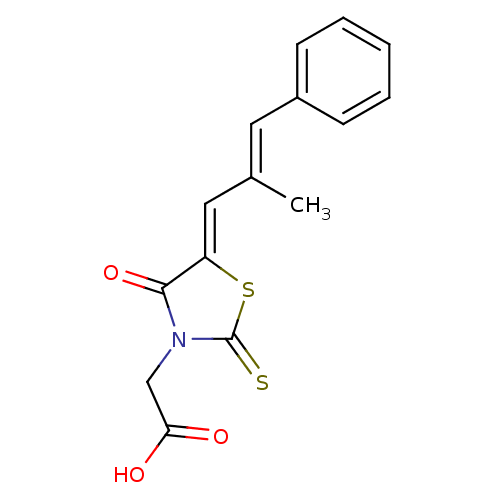
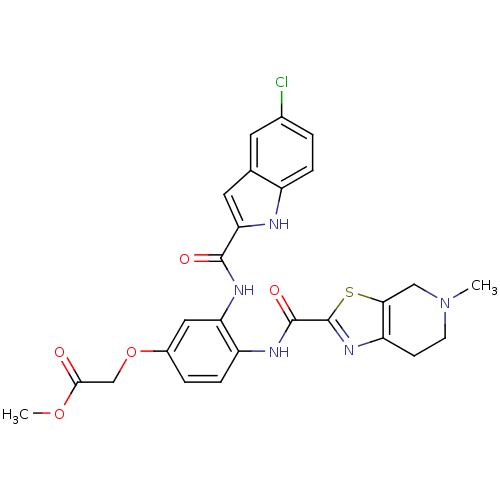
TargetAldo-keto reductase family 1 member B10(Homo sapiens (Human))
Gifu Pharmaceutical University
Curated by ChEMBL
Gifu Pharmaceutical University
Curated by ChEMBL
Affinity DataKi: 2.60nMAssay Description:Competitive inhibition at human recombinant N-terminus His6-tagged AKR1B10 expressed in Escherichia coli BL21 DE3 assessed as inhibition of NADP+ lin...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B10(Homo sapiens (Human))
Gifu Pharmaceutical University
Curated by ChEMBL
Gifu Pharmaceutical University
Curated by ChEMBL
Affinity DataKi: 15nMAssay Description:Inhibition of reductase activity of N-terminal 6His-tagged human AKR1B10 expressed in Escherichia coli BL21(DE3) assessed as pyridine-3-aldehyde redu...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B10(Homo sapiens (Human))
Gifu Pharmaceutical University
Curated by ChEMBL
Gifu Pharmaceutical University
Curated by ChEMBL
Affinity DataKi: 46nMAssay Description:Mixed-type inhibition at human recombinant N-terminus His6-tagged AKR1B10 expressed in Escherichia coli BL21 DE3 assessed as inhibition of NADP+ link...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B10(Homo sapiens (Human))
Gifu Pharmaceutical University
Curated by ChEMBL
Gifu Pharmaceutical University
Curated by ChEMBL
Affinity DataKi: 50nMAssay Description:Inhibition of reductase activity of N-terminal 6His-tagged human AKR1B10 expressed in Escherichia coli BL21(DE3) assessed as pyridine-3-aldehyde redu...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B10(Homo sapiens (Human))
Gifu Pharmaceutical University
Curated by ChEMBL
Gifu Pharmaceutical University
Curated by ChEMBL
Affinity DataKi: 72nMAssay Description:Competitive inhibition of dehydrogenase activity of N-terminal 6His-tagged human AKR1B10 expressed in Escherichia coli BL21(DE3) assessed as NADP+-li...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B10(Homo sapiens (Human))
Gifu Pharmaceutical University
Curated by ChEMBL
Gifu Pharmaceutical University
Curated by ChEMBL
Affinity DataKi: 2.00E+3nMAssay Description:Competitive inhibition of dehydrogenase activity of N-terminal 6His-tagged human AKR1B10 expressed in Escherichia coli BL21(DE3) assessed as NADP+-li...More data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:Inhibition of human factor 10a using S2222 chromogenic substrate by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.90nMAssay Description:Inhibition of human factor 10a using S2222 chromogenic substrate by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.90nMAssay Description:Inhibition of human factor 10a using S2222 chromogenic substrate by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 2.20nMAssay Description:Inhibition of human factor 10a using S2222 chromogenic substrate by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 2.5nMAssay Description:Inhibition of human factor 10a using S2222 chromogenic substrate by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 2.70nMAssay Description:Inhibition of human factor 10a using S2222 chromogenic substrate by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 3.5nMAssay Description:Inhibition of human factor 10a using S2222 chromogenic substrate by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 5.60nMAssay Description:Inhibition of human factor 10a using S2222 chromogenic substrate by spectrophotometric analysisMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B10(Homo sapiens (Human))
Gifu Pharmaceutical University
Curated by ChEMBL
Gifu Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 6.20nMAssay Description:Inhibition of human recombinant N-terminus His6-tagged AKR1B10 expressed in Escherichia coli BL21 DE3 assessed as pyridine-3-aldehyde reduction by sp...More data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4B(Homo sapiens (Human))
Asahi Kasei Pharma
Curated by ChEMBL
Asahi Kasei Pharma
Curated by ChEMBL
Affinity DataIC50: 6.80nMAssay Description:Inhibition of human PDE4B1 incubated for 10 mins using cAMP and [3H]cAMP substratesMore data for this Ligand-Target Pair
Affinity DataIC50: 7.40nMAssay Description:Inhibition of human factor 10a using S2222 chromogenic substrate by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 7.70nMAssay Description:Inhibition of human factor 10a using S2222 chromogenic substrate by spectrophotometric analysisMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B10(Homo sapiens (Human))
Gifu Pharmaceutical University
Curated by ChEMBL
Gifu Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 9nMAssay Description:Inhibition of human recombinant N-terminus His6-tagged AKR1B10 expressed in Escherichia coli BL21 DE3 assessed as pyridine-3-aldehyde reduction by sp...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B10(Homo sapiens (Human))
Gifu Pharmaceutical University
Curated by ChEMBL
Gifu Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Inhibition of human recombinant N-terminus His6-tagged AKR1B10 expressed in Escherichia coli BL21 DE3 assessed as pyridine-3-aldehyde reduction by sp...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B10(Homo sapiens (Human))
Gifu Pharmaceutical University
Curated by ChEMBL
Gifu Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Inhibition of human recombinant N-terminus His6-tagged AKR1B10 expressed in Escherichia coli BL21 DE3 assessed as pyridine-3-aldehyde reduction by sp...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B10(Homo sapiens (Human))
Gifu Pharmaceutical University
Curated by ChEMBL
Gifu Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 14nMAssay Description:Inhibition of human recombinant N-terminus His6-tagged AKR1B10 expressed in Escherichia coli BL21 DE3 assessed as pyridine-3-aldehyde reduction by sp...More data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4B(Homo sapiens (Human))
Asahi Kasei Pharma
Curated by ChEMBL
Asahi Kasei Pharma
Curated by ChEMBL
Affinity DataIC50: 15nMAssay Description:Inhibition of human PDE4B1 incubated for 10 mins using cAMP and [3H]cAMP substratesMore data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4B(Homo sapiens (Human))
Asahi Kasei Pharma
Curated by ChEMBL
Asahi Kasei Pharma
Curated by ChEMBL
Affinity DataIC50: 15nMAssay Description:Inhibition of human PDE4B1 incubated for 10 mins using cAMP and [3H]cAMP substratesMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B10(Homo sapiens (Human))
Gifu Pharmaceutical University
Curated by ChEMBL
Gifu Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 16nMAssay Description:Inhibition of human recombinant N-terminus His6-tagged AKR1B10 expressed in Escherichia coli BL21 DE3 assessed as pyridine-3-aldehyde reduction by sp...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B10(Homo sapiens (Human))
Gifu Pharmaceutical University
Curated by ChEMBL
Gifu Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 17nMAssay Description:Inhibition of human recombinant N-terminus His6-tagged AKR1B10 expressed in Escherichia coli BL21 DE3 assessed as pyridine-3-aldehyde reduction by sp...More data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4B(Homo sapiens (Human))
Asahi Kasei Pharma
Curated by ChEMBL
Asahi Kasei Pharma
Curated by ChEMBL
Affinity DataIC50: 19nMAssay Description:Inhibition of human PDE4B1 incubated for 10 mins using cAMP and [3H]cAMP substratesMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B10(Homo sapiens (Human))
Gifu Pharmaceutical University
Curated by ChEMBL
Gifu Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 21nMAssay Description:Inhibition of human recombinant N-terminus His6-tagged AKR1B10 expressed in Escherichia coli BL21 DE3 assessed as pyridine-3-aldehyde reduction by sp...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B10(Homo sapiens (Human))
Gifu Pharmaceutical University
Curated by ChEMBL
Gifu Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 23nMAssay Description:Inhibition of human recombinant N-terminus His6-tagged AKR1B10 expressed in Escherichia coli BL21 DE3 assessed as pyridine-3-aldehyde reduction by sp...More data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4D(Homo sapiens (Human))
Asahi Kasei Pharma
Curated by ChEMBL
Asahi Kasei Pharma
Curated by ChEMBL
Affinity DataIC50: 27nMAssay Description:Inhibition of human PDE4D3 incubated for 10 mins using cAMP and [3H]cAMP substratesMore data for this Ligand-Target Pair
Affinity DataIC50: 29nMAssay Description:Inhibition of human factor 10a using S2222 chromogenic substrate by spectrophotometric analysisMore data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4B(Homo sapiens (Human))
Asahi Kasei Pharma
Curated by ChEMBL
Asahi Kasei Pharma
Curated by ChEMBL
Affinity DataIC50: 34nMAssay Description:Inhibition of human PDE4B1 incubated for 10 mins using cAMP and [3H]cAMP substratesMore data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4B(Homo sapiens (Human))
Asahi Kasei Pharma
Curated by ChEMBL
Asahi Kasei Pharma
Curated by ChEMBL
Affinity DataIC50: 34nMAssay Description:Inhibition of human PDE4B1 incubated for 10 mins using cAMP and [3H]cAMP substratesMore data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4B(Homo sapiens (Human))
Asahi Kasei Pharma
Curated by ChEMBL
Asahi Kasei Pharma
Curated by ChEMBL
Affinity DataIC50: 37nMAssay Description:Inhibition of human PDE4B1 incubated for 10 mins using cAMP and [3H]cAMP substratesMore data for this Ligand-Target Pair
Affinity DataIC50: 47nMAssay Description:Inhibition of human factor 10a using S2222 chromogenic substrate by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 65nMAssay Description:Inhibition of human factor 10a using S2222 chromogenic substrate by spectrophotometric analysisMore data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4B(Homo sapiens (Human))
Asahi Kasei Pharma
Curated by ChEMBL
Asahi Kasei Pharma
Curated by ChEMBL
Affinity DataIC50: 68nMAssay Description:Inhibition of human PDE4B1 incubated for 10 mins using cAMP and [3H]cAMP substratesMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B10(Homo sapiens (Human))
Gifu Pharmaceutical University
Curated by ChEMBL
Gifu Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 69nMAssay Description:Inhibition of human recombinant N-terminus His6-tagged AKR1B10 expressed in Escherichia coli BL21 DE3 assessed as pyridine-3-aldehyde reduction by sp...More data for this Ligand-Target Pair
Affinity DataIC50: 70nMAssay Description:Inhibition of human factor 10a using S2222 chromogenic substrate by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 72nMAssay Description:Inhibition of human factor 10a using S2222 chromogenic substrate by spectrophotometric analysisMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Homo sapiens (Human))
Gifu Pharmaceutical University
Curated by ChEMBL
Gifu Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 78nMAssay Description:Inhibition of human recombinant N-terminus His6-tagged AKR1B1 expressed in Escherichia coli BL21 DE3 assessed as pyridine-3-aldehyde reduction by spe...More data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4B(Homo sapiens (Human))
Asahi Kasei Pharma
Curated by ChEMBL
Asahi Kasei Pharma
Curated by ChEMBL
Affinity DataIC50: 78nMAssay Description:Inhibition of human PDE4B1 incubated for 10 mins using cAMP and [3H]cAMP substratesMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B10(Homo sapiens (Human))
Gifu Pharmaceutical University
Curated by ChEMBL
Gifu Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 80nMAssay Description:Inhibition of human recombinant N-terminus His6-tagged AKR1B10 expressed in Escherichia coli BL21 DE3 assessed as pyridine-3-aldehyde reduction by sp...More data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4D(Homo sapiens (Human))
Asahi Kasei Pharma
Curated by ChEMBL
Asahi Kasei Pharma
Curated by ChEMBL
Affinity DataIC50: 82nMAssay Description:Inhibition of human PDE4D3 incubated for 10 mins using cAMP and [3H]cAMP substratesMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Homo sapiens (Human))
Gifu Pharmaceutical University
Curated by ChEMBL
Gifu Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 88nMAssay Description:Inhibition of human recombinant N-terminus His6-tagged AKR1B1 expressed in Escherichia coli BL21 DE3 assessed as pyridine-3-aldehyde reduction by spe...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B10(Homo sapiens (Human))
Gifu Pharmaceutical University
Curated by ChEMBL
Gifu Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 90nMAssay Description:Inhibition of reductase activity of N-terminal 6His-tagged human AKR1B10 expressed in Escherichia coli BL21(DE3) assessed as pyridine-3-aldehyde redu...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Homo sapiens (Human))
Gifu Pharmaceutical University
Curated by ChEMBL
Gifu Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 100nMAssay Description:Inhibition of human recombinant AKR1B1 expressed in Escherichia coli BL21 cells using pyridine-3-aldehyde as substrate by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Inhibition of human factor 10a using S2222 chromogenic substrate by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Inhibition of human factor 10a using S2222 chromogenic substrate by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 110nMAssay Description:Inhibition of human factor 10a using S2222 chromogenic substrate by spectrophotometric analysisMore data for this Ligand-Target Pair


 3D Structure (crystal)
3D Structure (crystal)