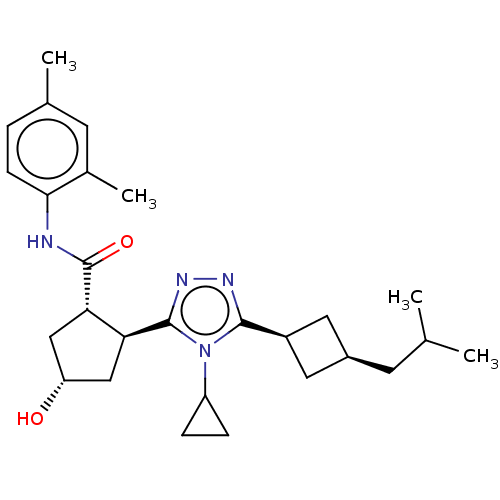
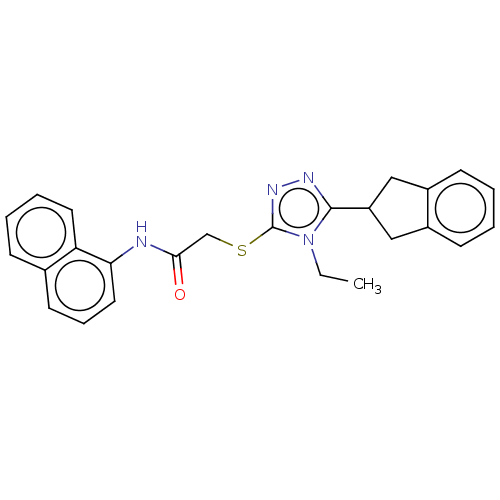
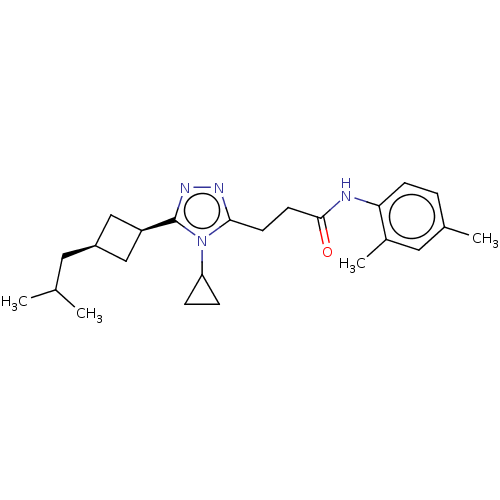
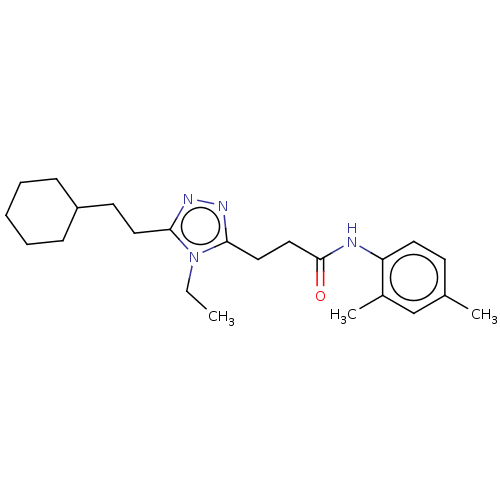
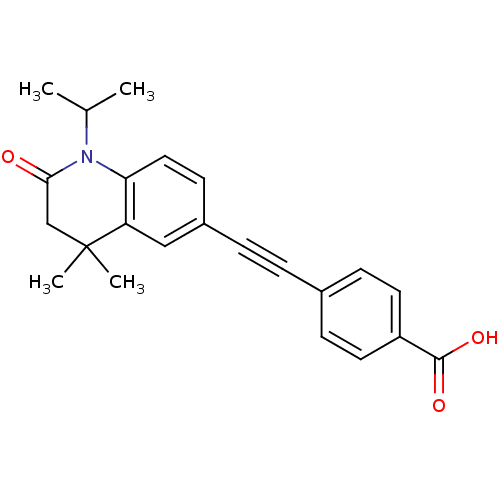
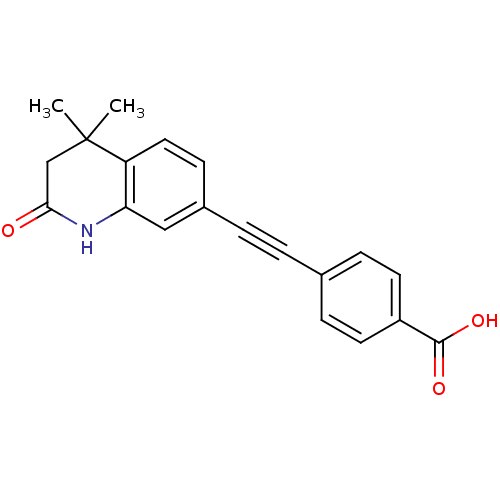
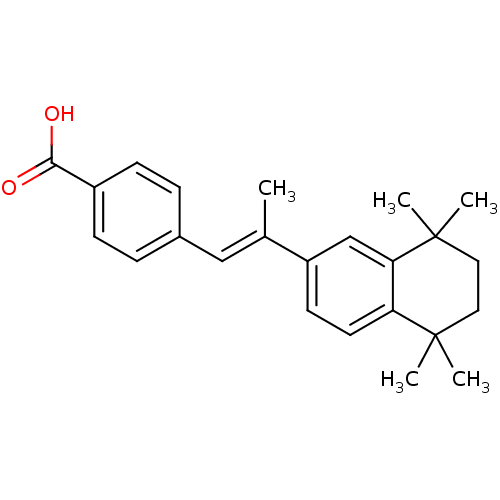
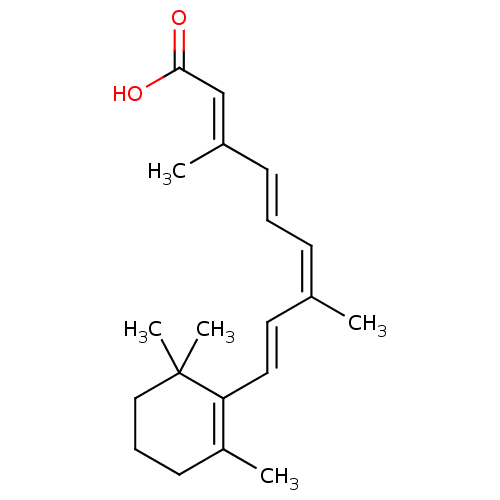
TargetDihydroorotate dehydrogenase (quinone), mitochondrial(Homo sapiens (Human))
Allergan
Curated by ChEMBL
Allergan
Curated by ChEMBL
Affinity DataKi: 2.70E+3nMAssay Description:Inhibition of dihydroorotate dehydrogenaseMore data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate preincubated with substrate for 5 mins followed by NADPH addition measure...More data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 4.00E+3nMAssay Description:Inhibition of human CYP3A4More data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate preincubated with substrate for 5 mins followed by NADPH addition measure...More data for this Ligand-Target Pair
Affinity DataIC50: 7.00E+3nMAssay Description:Inhibition of EGF-dependent proliferation of human and guinea pig keratinocytes; range 7-15 uMMore data for this Ligand-Target Pair
Affinity DataIC50: 7.00E+3nMAssay Description:Inhibition of EGF-dependent proliferation of human and guinea pig keratinocytes; range 7-15 uMMore data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.20E+4nMAssay Description:Inhibition of human CYP3A4More data for this Ligand-Target Pair
TargetCytochrome P450 2C9(Homo sapiens (Human))
Kyoto Prefectural University Of Medicine
Curated by ChEMBL
Kyoto Prefectural University Of Medicine
Curated by ChEMBL
Affinity DataIC50: >5.00E+4nMAssay Description:Time-dependent inhibition of human CYP2C9More data for this Ligand-Target Pair
TargetCytochrome P450 2D6(Homo sapiens (Human))
Kyoto Prefectural University Of Medicine
Curated by ChEMBL
Kyoto Prefectural University Of Medicine
Curated by ChEMBL
Affinity DataIC50: >5.00E+4nMAssay Description:Time-dependent inhibition of human CYP2D6More data for this Ligand-Target Pair
TargetCytochrome P450 2C19(Homo sapiens (Human))
Kyoto Prefectural University Of Medicine
Curated by ChEMBL
Kyoto Prefectural University Of Medicine
Curated by ChEMBL
Affinity DataIC50: >5.00E+4nMAssay Description:Time-dependent inhibition of human CYP2C19More data for this Ligand-Target Pair
TargetCytochrome P450 1A2(Homo sapiens (Human))
Kyoto Prefectural University Of Medicine
Curated by ChEMBL
Kyoto Prefectural University Of Medicine
Curated by ChEMBL
Affinity DataIC50: >5.00E+4nMAssay Description:Time-dependent inhibition of human CYP1A2More data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: >5.00E+4nMAssay Description:Time-dependent inhibition of human CYP3A4 using midazolam as substrateMore data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate preincubated with substrate for 5 mins followed by NADPH addition measure...More data for this Ligand-Target Pair
TargetCytochrome P450 2C9(Homo sapiens (Human))
Kyoto Prefectural University Of Medicine
Curated by ChEMBL
Kyoto Prefectural University Of Medicine
Curated by ChEMBL
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of CYP2C9 in human liver microsomes using diclofenac as substrate preincubated with substrate for 5 mins followed by NADPH addition measur...More data for this Ligand-Target Pair
TargetCytochrome P450 2D6(Homo sapiens (Human))
Kyoto Prefectural University Of Medicine
Curated by ChEMBL
Kyoto Prefectural University Of Medicine
Curated by ChEMBL
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of CYP2D6 in human liver microsomes using bufuralol as substrate preincubated with substrate for 5 mins followed by NADPH addition measure...More data for this Ligand-Target Pair
TargetCytochrome P450 1A2(Homo sapiens (Human))
Kyoto Prefectural University Of Medicine
Curated by ChEMBL
Kyoto Prefectural University Of Medicine
Curated by ChEMBL
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of CYP1A2 in human liver microsomes using ethoxyresorufin as substrate preincubated with substrate for 5 mins followed by NADPH addition m...More data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: >5.00E+4nMAssay Description:Time-dependent inhibition of human CYP3A4 using testosterone as substrateMore data for this Ligand-Target Pair
TargetCytochrome P450 2C19(Homo sapiens (Human))
Kyoto Prefectural University Of Medicine
Curated by ChEMBL
Kyoto Prefectural University Of Medicine
Curated by ChEMBL
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of CYP2C19 in human liver microsomes using S-mephenytoin as substrate preincubated with substrate for 5 mins followed by NADPH addition me...More data for this Ligand-Target Pair
TargetCytochrome P450 2A6(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of CYP2A6 in human liver microsomes using coumarin as substrate preincubated with substrate for 5 mins followed by NADPH addition measured...More data for this Ligand-Target Pair
Affinity DataKd: 18nMAssay Description:Binding affinity of the compound was determined for Retinoic acid receptor betaMore data for this Ligand-Target Pair
Affinity DataEC50: 17nMAssay Description:Transactivation potency of the compound was determined for Retinoic acid receptor alpha; Not activeMore data for this Ligand-Target Pair
Affinity DataKd: 18nMAssay Description:Binding affinity of the compound was determined for Retinoic acid receptor betaMore data for this Ligand-Target Pair
Affinity DataKd: >1.00E+3nMAssay Description:Binding affinity of the compound was determined for Retinoic acid receptor betaMore data for this Ligand-Target Pair
Affinity DataEC50: 350nMAssay Description:Transactivation potency of the compound was determined for Retinoic acid receptor alphaMore data for this Ligand-Target Pair
Affinity DataEC50: 20nMAssay Description:Transactivation potency of the compound was determined for Retinoic acid receptor gammaMore data for this Ligand-Target Pair
Affinity DataEC50: 36nMAssay Description:Transactivation potency of the compound was determined for Retinoic acid receptor betaMore data for this Ligand-Target Pair
Affinity DataEC50: 6nMAssay Description:Transactivation potency of the compound was determined for Retinoic acid receptor gammaMore data for this Ligand-Target Pair
Affinity DataEC50: 4nMAssay Description:Transactivation potency of the compound was determined for Retinoic acid receptor gammaMore data for this Ligand-Target Pair
Affinity DataEC50: 1.00E+3nMAssay Description:Transactivation potency of the compound was determined for Retinoic acid receptor betaMore data for this Ligand-Target Pair
Affinity DataEC50: 30nMAssay Description:Transactivation potency of the compound was determined for Retinoic acid receptor alphaMore data for this Ligand-Target Pair
Affinity DataEC50: 2.30E+3nMAssay Description:Transactivation potency of the compound was determined for Retinoic acid receptor alphaMore data for this Ligand-Target Pair
Affinity DataEC50: 200nMAssay Description:Transactivation potency of the compound was determined for Retinoic acid receptor gammaMore data for this Ligand-Target Pair
Affinity DataEC50: 54nMAssay Description:Transactivation potency of the compound was determined for Retinoic acid receptor gammaMore data for this Ligand-Target Pair
Affinity DataKd: 17nMAssay Description:Binding affinity of the compound was determined for Retinoic acid receptor betaMore data for this Ligand-Target Pair
Affinity DataKd: 18nMAssay Description:Binding affinity for Retinoic acid receptor gammaMore data for this Ligand-Target Pair
Affinity DataEC50: 170nMAssay Description:Transactivation potency of the compound was determined for Retinoic acid receptor betaMore data for this Ligand-Target Pair
Affinity DataEC50: 260nMAssay Description:Transactivation potency of the compound was determined for Retinoic acid receptor gammaMore data for this Ligand-Target Pair
Affinity DataKd: 100nMAssay Description:Inhibition of [3H]-ATRA binding to baculovirus expressed retinoic acid receptor RAR-betaMore data for this Ligand-Target Pair
Affinity DataEC50: 1.5nMAssay Description:Transcriptional activation in CV-1 cells expressing retinoid X receptor RXR alphaMore data for this Ligand-Target Pair
Affinity DataKd: 2.5nMAssay Description:Inhibition of [3H]-9-cis-retinoic acid binding to baculovirus expressed retinoic acid receptor RXR-betaMore data for this Ligand-Target Pair
Affinity DataEC50: 29nMAssay Description:Transcriptional activation in CV-1 cells expressing retinoid X receptor RXR betaMore data for this Ligand-Target Pair
Affinity DataEC50: 4nMAssay Description:Transcriptional activation in CV-1 cells expressing retinoic acid receptor RAR gammaMore data for this Ligand-Target Pair
Affinity DataKd: 35nMAssay Description:Inhibition of [3H]-9-cis-retinoic acid binding to baculovirus expressed retinoic acid receptor RXR-betaMore data for this Ligand-Target Pair
Affinity DataEC50: 0.800nMAssay Description:Transcriptional activation in CV-1 cells expressing retinoid X receptor RXR betaMore data for this Ligand-Target Pair
Affinity DataEC50: 0.0800nMAssay Description:Transcriptional activation in CV-1 cells expressing retinoid X receptor RXR gammaMore data for this Ligand-Target Pair
Affinity DataKd: 1.5nMAssay Description:Inhibition of [3H]-9-cis-retinoic acid binding to baculovirus expressed retinoic acid receptor RXR-alphaMore data for this Ligand-Target Pair
Affinity DataKd: 6.20E+3nMAssay Description:Inhibition of [3H]-ATRA binding to baculovirus expressed retinoic acid receptor RAR-betaMore data for this Ligand-Target Pair
Affinity DataEC50: >500nMAssay Description:Transcriptional activation in CV-1 cells expressing retinoic acid receptor RAR betaMore data for this Ligand-Target Pair
Affinity DataKd: >10nMAssay Description:Inhibition of [3H]-ATRA binding to baculovirus expressed retinoic acid receptor RAR-gammaMore data for this Ligand-Target Pair
Affinity DataKd: 13nMAssay Description:Inhibition of [3H]-9-cis-retinoic acid binding to baculovirus expressed retinoic acid receptor RXR-alphaMore data for this Ligand-Target Pair

 3D Structure (crystal)
3D Structure (crystal)