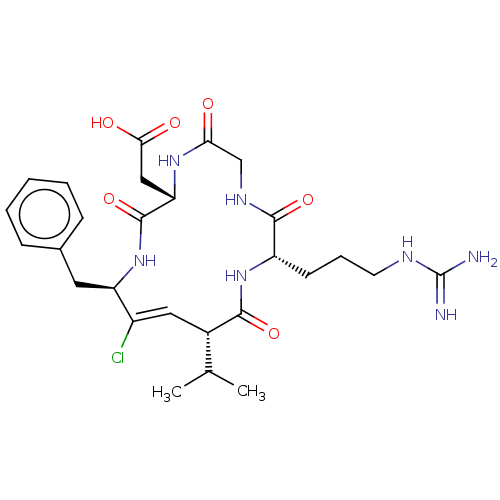
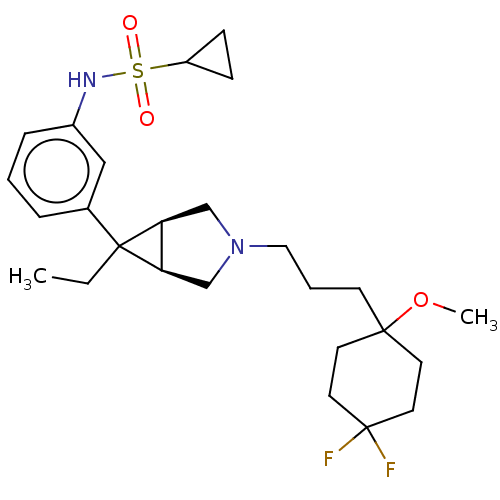
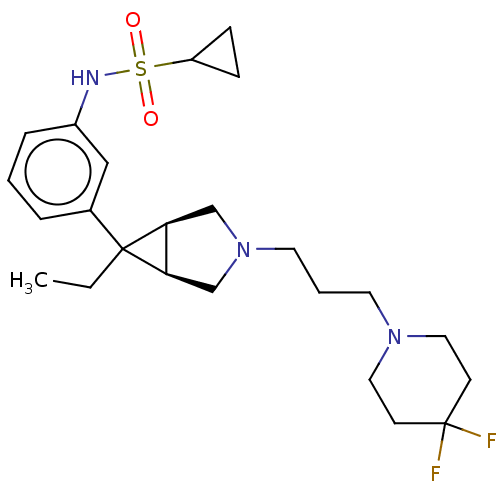
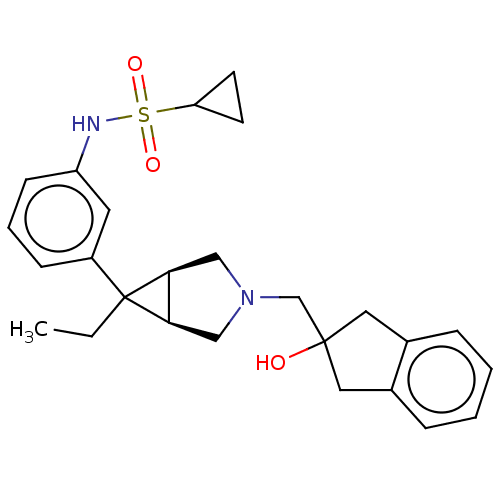
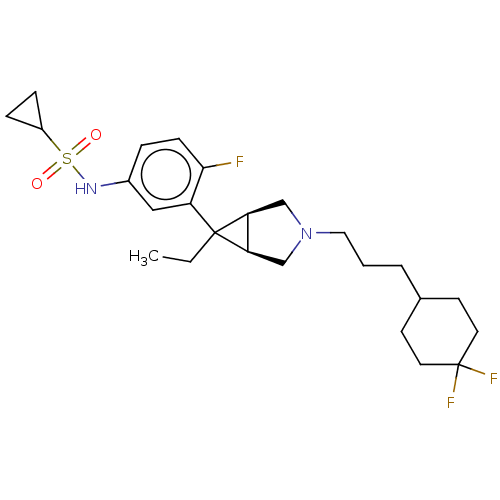
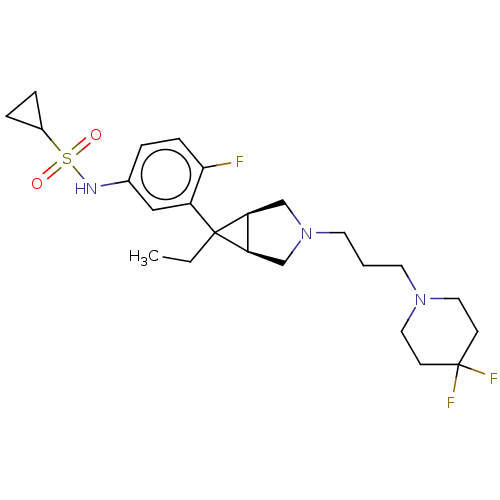
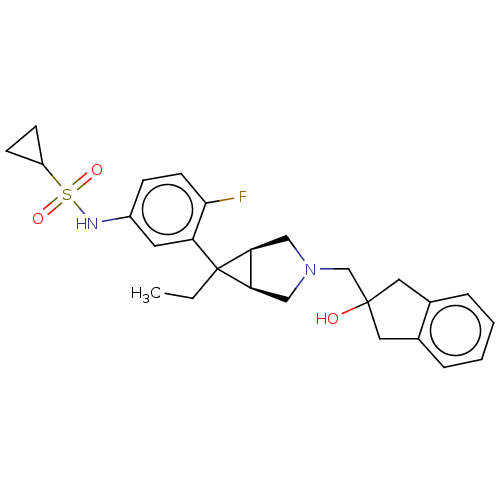
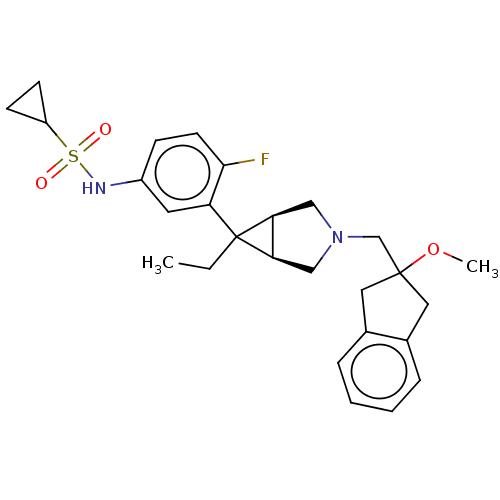
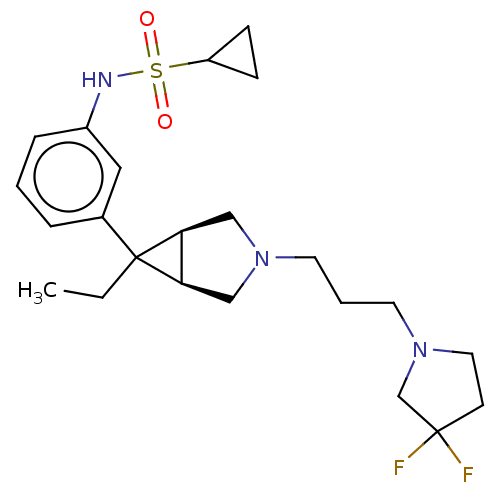
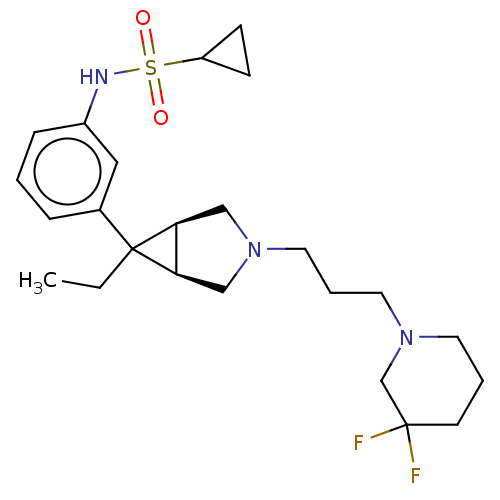
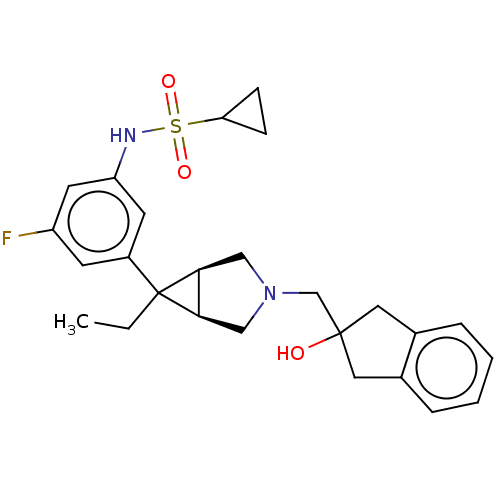
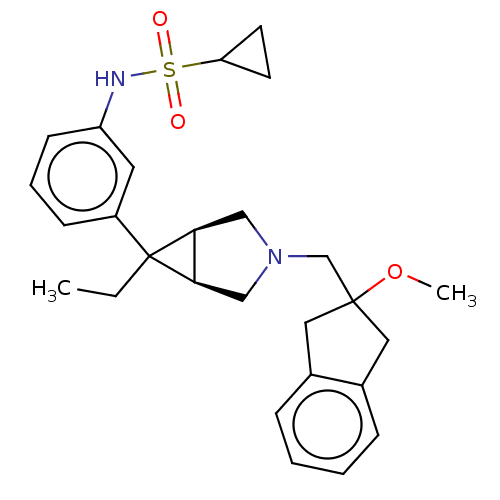
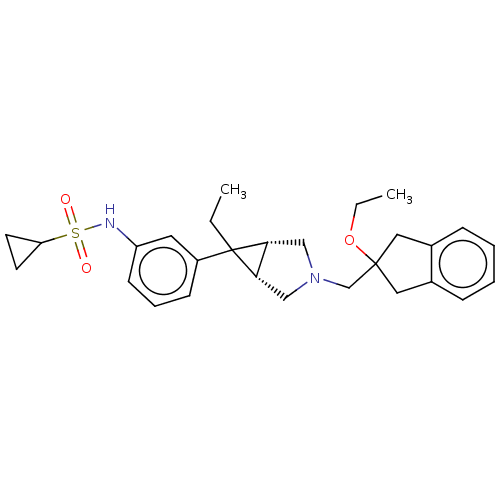
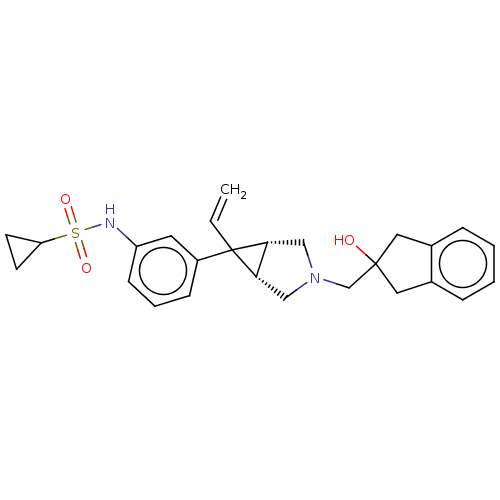
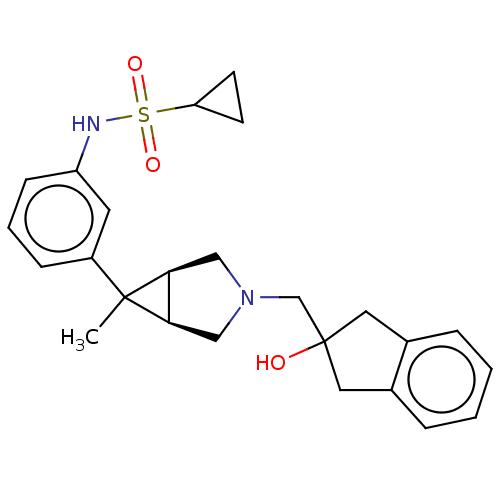
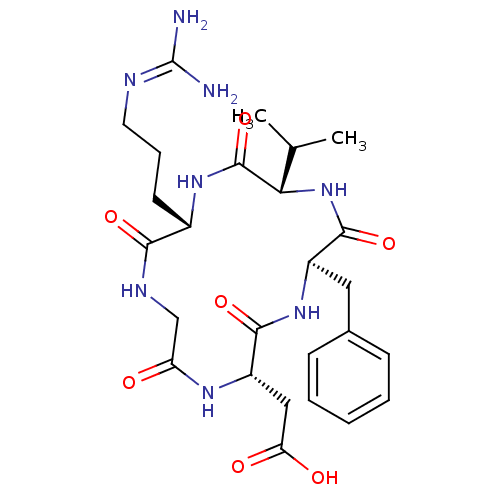
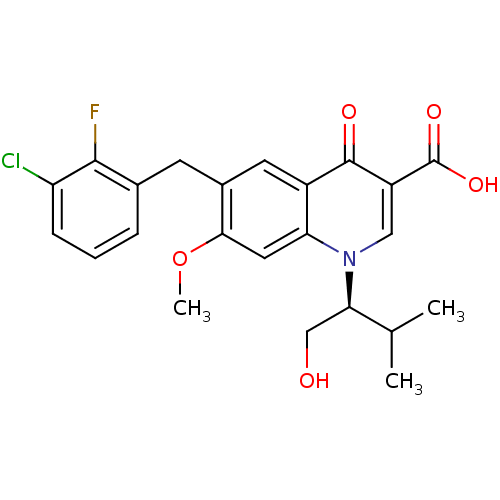
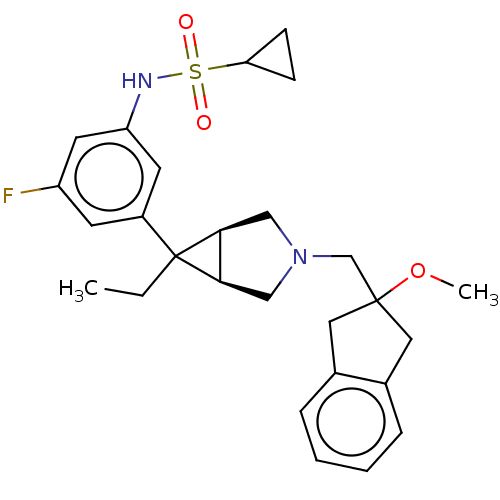
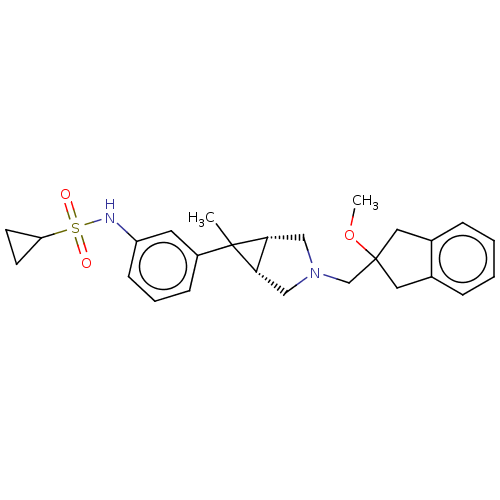
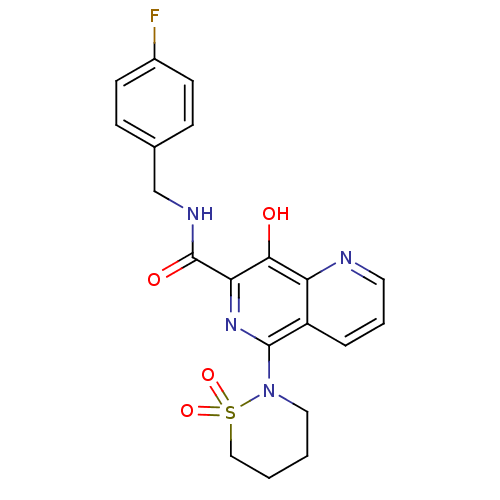
TargetIntegrin alpha-V/beta-3(Homo sapiens (Human))
Tokyo Medical And Dental University
Curated by ChEMBL
Tokyo Medical And Dental University
Curated by ChEMBL
Affinity DataIC50: 0.497nMAssay Description:Inhibition of vitronectin binding to integrin alphaVbeta3 in HDF pretreated for 20 mins followed by vitronectin addition measured after 40 mins by cr...More data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMAssay Description:The inhibition ratio of the test substance at the respective concentrations were calculated with setting the reaction value of the well to which DMSO...More data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMAssay Description:The inhibition ratio of the test substance at the respective concentrations were calculated with setting the reaction value of the well to which DMSO...More data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMAssay Description:The inhibition ratio of the test substance at the respective concentrations were calculated with setting the reaction value of the well to which DMSO...More data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:The inhibition ratio of the test substance at the respective concentrations were calculated with setting the reaction value of the well to which DMSO...More data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:The inhibition ratio of the test substance at the respective concentrations were calculated with setting the reaction value of the well to which DMSO...More data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:The inhibition ratio of the test substance at the respective concentrations were calculated with setting the reaction value of the well to which DMSO...More data for this Ligand-Target Pair
Affinity DataIC50: 1.60nMAssay Description:The inhibition ratio of the test substance at the respective concentrations were calculated with setting the reaction value of the well to which DMSO...More data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMAssay Description:The inhibition ratio of the test substance at the respective concentrations were calculated with setting the reaction value of the well to which DMSO...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:The inhibition ratio of the test substance at the respective concentrations were calculated with setting the reaction value of the well to which DMSO...More data for this Ligand-Target Pair
TargetIntegrin alpha-V/beta-3(Homo sapiens (Human))
Tokyo Medical And Dental University
Curated by ChEMBL
Tokyo Medical And Dental University
Curated by ChEMBL
Affinity DataIC50: 2.40nMAssay Description:Inhibition of vitronectin binding to human integrin alphaVbeta3 measured after 4 hrs by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 2.5nMAssay Description:The inhibition ratio of the test substance at the respective concentrations were calculated with setting the reaction value of the well to which DMSO...More data for this Ligand-Target Pair
Affinity DataIC50: 2.80nMAssay Description:The inhibition ratio of the test substance at the respective concentrations were calculated with setting the reaction value of the well to which DMSO...More data for this Ligand-Target Pair
Affinity DataIC50: 2.90nMAssay Description:The inhibition ratio of the test substance at the respective concentrations were calculated with setting the reaction value of the well to which DMSO...More data for this Ligand-Target Pair
Affinity DataIC50: 3.10nMAssay Description:The inhibition ratio of the test substance at the respective concentrations were calculated with setting the reaction value of the well to which DMSO...More data for this Ligand-Target Pair
Affinity DataIC50: 3.20nMAssay Description:The inhibition ratio of the test substance at the respective concentrations were calculated with setting the reaction value of the well to which DMSO...More data for this Ligand-Target Pair
Affinity DataIC50: 3.60nMAssay Description:The inhibition ratio of the test substance at the respective concentrations were calculated with setting the reaction value of the well to which DMSO...More data for this Ligand-Target Pair
TargetIntegrin alpha-V/beta-3(Homo sapiens (Human))
Tokyo Medical And Dental University
Curated by ChEMBL
Tokyo Medical And Dental University
Curated by ChEMBL
Affinity DataIC50: 3.60nMAssay Description:Inhibition of vitronectin binding to human integrin alphaVbeta3 measured after 4 hrs by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 4.10nMAssay Description:The inhibition ratio of the test substance at the respective concentrations were calculated with setting the reaction value of the well to which DMSO...More data for this Ligand-Target Pair
Affinity DataIC50: 4.10nMAssay Description:The inhibition ratio of the test substance at the respective concentrations were calculated with setting the reaction value of the well to which DMSO...More data for this Ligand-Target Pair
Affinity DataIC50: 4.5nMAssay Description:The inhibition ratio of the test substance at the respective concentrations were calculated with setting the reaction value of the well to which DMSO...More data for this Ligand-Target Pair
Affinity DataIC50: 5.40nMAssay Description:The inhibition ratio of the test substance at the respective concentrations were calculated with setting the reaction value of the well to which DMSO...More data for this Ligand-Target Pair
TargetIntegrin alpha-V/beta-3(Homo sapiens (Human))
Tokyo Medical And Dental University
Curated by ChEMBL
Tokyo Medical And Dental University
Curated by ChEMBL
Affinity DataIC50: 6.80nMAssay Description:Inhibition of vitronectin binding to human integrin alphaVbeta3 measured after 4 hrs by ELISAMore data for this Ligand-Target Pair
TargetIntegrase(Human immunodeficiency virus 1)
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 7.20nMAssay Description:Inhibition of recombinant HIV1 integrase strand transfer activityMore data for this Ligand-Target Pair
TargetIntegrase(Human immunodeficiency virus 1)
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 8.20nMAssay Description:Inhibition of recombinant HIV1 integrase strand transfer activityMore data for this Ligand-Target Pair
Affinity DataIC50: 9nMAssay Description:Inhibition of HIV1 integrase strand transfer activityMore data for this Ligand-Target Pair
TargetIntegrase(Human immunodeficiency virus 1)
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 9.10nMAssay Description:Inhibition of recombinant HIV1 integrase strand transfer activityMore data for this Ligand-Target Pair
TargetIntegrin alpha-V/beta-3(Homo sapiens (Human))
Tokyo Medical And Dental University
Curated by ChEMBL
Tokyo Medical And Dental University
Curated by ChEMBL
Affinity DataIC50: 11nMAssay Description:Inhibition of vitronectin binding to integrin alphaVbeta3 in HDF pretreated for 20 mins followed by vitronectin addition measured after 40 mins by cr...More data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:Inhibition of HIV1 integrase strand transfer activityMore data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:The inhibition ratio of the test substance at the respective concentrations were calculated with setting the reaction value of the well to which DMSO...More data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:The inhibition ratio of the test substance at the respective concentrations were calculated with setting the reaction value of the well to which DMSO...More data for this Ligand-Target Pair
Affinity DataIC50: 21nMAssay Description:Inhibition of HIV1 integrase strand transfer activityMore data for this Ligand-Target Pair
TargetIntegrase(Human immunodeficiency virus 1)
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 23nMAssay Description:Inhibition of recombinant HIV1 integrase strand transfer activityMore data for this Ligand-Target Pair
Affinity DataIC50: 24nMAssay Description:Inhibition of HIV1 integrase strand transfer activityMore data for this Ligand-Target Pair
TargetIntegrase(Human immunodeficiency virus 1)
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 24nMAssay Description:Inhibition of recombinant HIV1 integrase strand transfer activityMore data for this Ligand-Target Pair
Affinity DataIC50: 25nMAssay Description:Inhibition of HIV1 integrase strand transfer activityMore data for this Ligand-Target Pair
Affinity DataIC50: 26nMAssay Description:Inhibition of HIV1 integrase strand transfer activityMore data for this Ligand-Target Pair
Affinity DataIC50: 31nMAssay Description:Inhibition of HIV1 integrase strand transfer activityMore data for this Ligand-Target Pair
Affinity DataIC50: 32nMAssay Description:Inhibition of HIV1 integrase strand transfer activityMore data for this Ligand-Target Pair
Affinity DataIC50: 34nMAssay Description:Inhibition of HIV1 integrase strand transfer activityMore data for this Ligand-Target Pair
Affinity DataIC50: 35nMAssay Description:Inhibition of HIV1 integrase strand transfer activityMore data for this Ligand-Target Pair
Affinity DataIC50: 38nMAssay Description:Inhibition of HIV1 integrase strand transfer activityMore data for this Ligand-Target Pair
Affinity DataIC50: 41nMAssay Description:Inhibition of HIV1 integrase strand transfer activityMore data for this Ligand-Target Pair
Affinity DataIC50: 43nMAssay Description:Inhibition of HIV1 integrase strand transfer activityMore data for this Ligand-Target Pair
TargetIntegrase(Human immunodeficiency virus 1)
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 44nMAssay Description:Inhibition of recombinant HIV1 integrase strand transfer activityMore data for this Ligand-Target Pair
Affinity DataIC50: 44nMAssay Description:Inhibition of HIV1 integrase strand transfer activityMore data for this Ligand-Target Pair
Affinity DataIC50: 50nMAssay Description:Inhibition of HIV1 integrase strand transfer activityMore data for this Ligand-Target Pair
Affinity DataIC50: 50nMAssay Description:Inhibition of HIV1 integrase strand transfer activityMore data for this Ligand-Target Pair
Affinity DataIC50: 55nMAssay Description:Inhibition of HIV1 integrase strand transfer activityMore data for this Ligand-Target Pair
Affinity DataIC50: 65nMAssay Description:Inhibition of HIV1 integrase strand transfer activityMore data for this Ligand-Target Pair