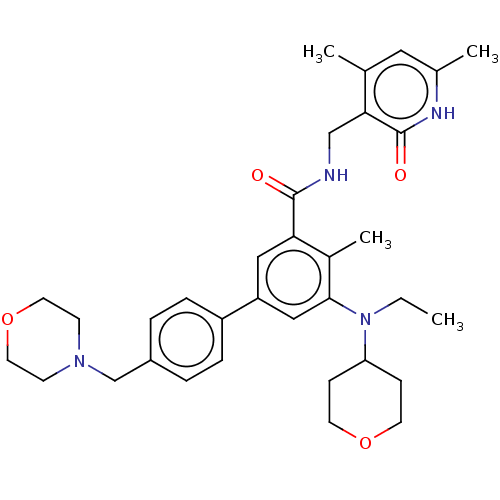
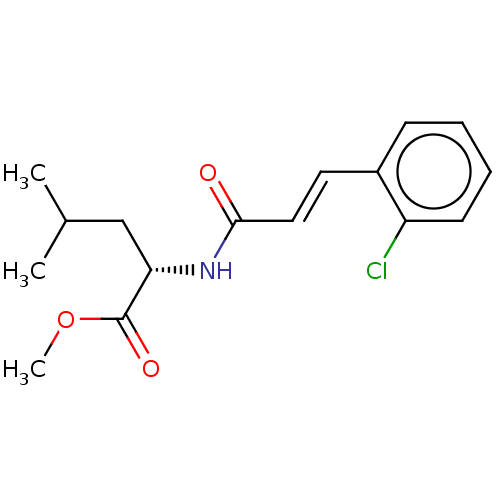
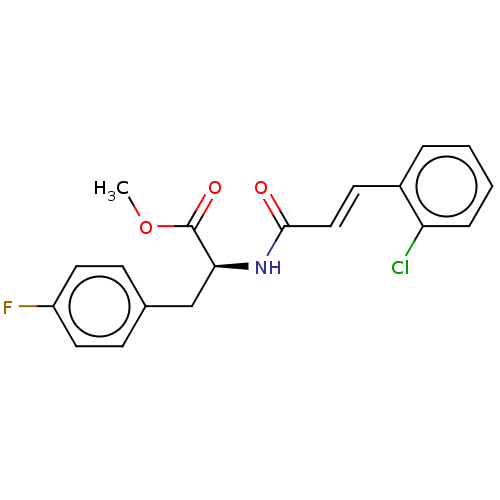
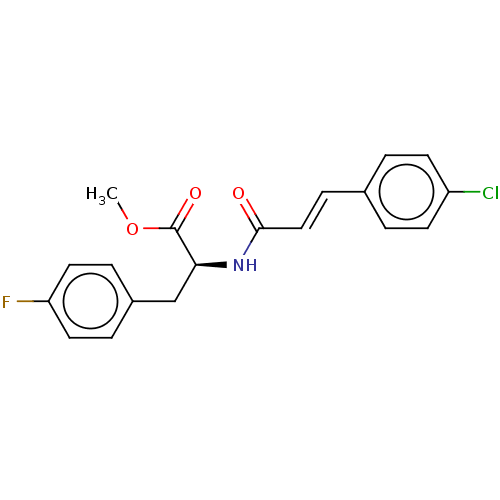
Affinity DataKi: 2.75E+3nM ΔG°: -32.3kJ/molepH: 8.0 T: 2°CAssay Description:Inhibition of E. coli KARI (ketol-acid reductoisomerase) is time dependent, and activity was measured by the decrease in A340 at 30 C in solutions co...More data for this Ligand-Target Pair
Affinity DataKi: 3.12E+4nM ΔG°: -26.2kJ/molepH: 8.0 T: 2°CAssay Description:Inhibition of E. coli KARI (ketol-acid reductoisomerase) is time dependent, and activity was measured by the decrease in A340 at 30 C in solutions co...More data for this Ligand-Target Pair
Affinity DataKi: 7.66E+4nM ΔG°: -23.9kJ/molepH: 8.0 T: 2°CAssay Description:Inhibition of E. coli KARI (ketol-acid reductoisomerase) is time dependent, and activity was measured by the decrease in A340 at 30 C in solutions co...More data for this Ligand-Target Pair
Affinity DataKi: 9.53E+4nM ΔG°: -23.3kJ/molepH: 8.0 T: 2°CAssay Description:Inhibition of E. coli KARI (ketol-acid reductoisomerase) is time dependent, and activity was measured by the decrease in A340 at 30 C in solutions co...More data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Homo sapiens (Human))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 8.40nMAssay Description:Inhibition of human iNOS assessed as reduction in L-[3H]-citrulline level using L-[3H]-arginine as substrate incubated for 1 hrMore data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2 [Y641F](Homo sapiens (Human))
Tarapeutics Science
US Patent
Tarapeutics Science
US Patent
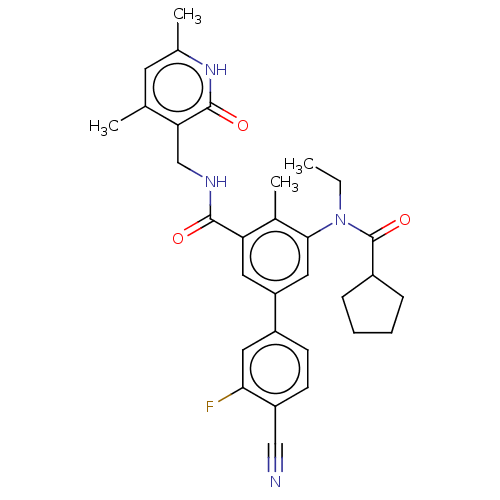
Affinity DataIC50: 16nMAssay Description:Enzymatic activity assay was conducted using EZH2(Y641F) TR-FRET assay KIT from Cisbio company on compounds that were shown to be active in primary s...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2 [Y641F](Homo sapiens (Human))
Tarapeutics Science
US Patent
Tarapeutics Science
US Patent
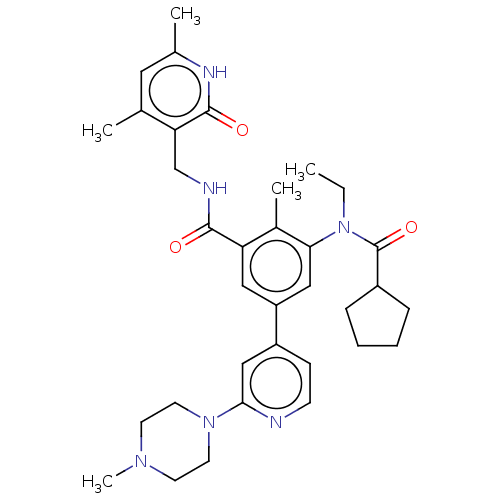
Affinity DataIC50: 20nMAssay Description:Enzymatic activity assay was conducted using EZH2(Y641F) TR-FRET assay KIT from Cisbio company on compounds that were shown to be active in primary s...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2 [Y641F](Homo sapiens (Human))
Tarapeutics Science
US Patent
Tarapeutics Science
US Patent
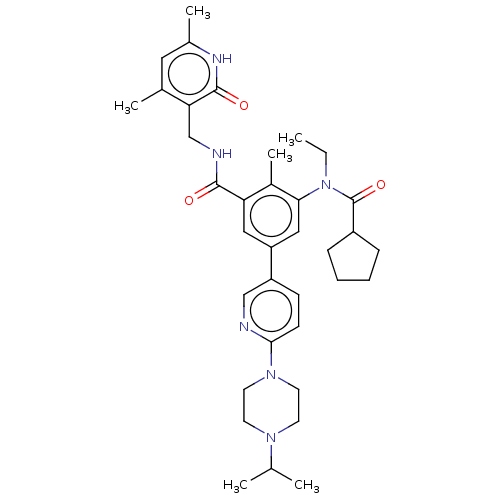
Affinity DataIC50: 23nMAssay Description:Enzymatic activity assay was conducted using EZH2(Y641F) TR-FRET assay KIT from Cisbio company on compounds that were shown to be active in primary s...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2 [Y641F](Homo sapiens (Human))
Tarapeutics Science
US Patent
Tarapeutics Science
US Patent
Affinity DataIC50: 29nMAssay Description:Enzymatic activity assay was conducted using EZH2(Y641F) TR-FRET assay KIT from Cisbio company on compounds that were shown to be active in primary s...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2 [Y641F](Homo sapiens (Human))
Tarapeutics Science
US Patent
Tarapeutics Science
US Patent
Affinity DataIC50: 58.6nMAssay Description:Enzymatic activity assay was conducted using EZH2(Y641F) TR-FRET assay KIT from Cisbio company on compounds that were shown to be active in primary s...More data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Homo sapiens (Human))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 74nMAssay Description:Inhibition of human iNOS assessed as reduction in L-[3H]-citrulline level using L-[3H]-arginine as substrate incubated for 1 hrMore data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2 [Y641F](Homo sapiens (Human))
Tarapeutics Science
US Patent
Tarapeutics Science
US Patent
Affinity DataIC50: 75nMAssay Description:Enzymatic activity assay was conducted using EZH2(Y641F) TR-FRET assay KIT from Cisbio company on compounds that were shown to be active in primary s...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2 [Y641F](Homo sapiens (Human))
Tarapeutics Science
US Patent
Tarapeutics Science
US Patent
Affinity DataIC50: 80.6nMAssay Description:Enzymatic activity assay was conducted using EZH2(Y641F) TR-FRET assay KIT from Cisbio company on compounds that were shown to be active in primary s...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2 [Y641F](Homo sapiens (Human))
Tarapeutics Science
US Patent
Tarapeutics Science
US Patent
Affinity DataIC50: 103nMAssay Description:Enzymatic activity assay was conducted using EZH2(Y641F) TR-FRET assay KIT from Cisbio company on compounds that were shown to be active in primary s...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2 [Y641F](Homo sapiens (Human))
Tarapeutics Science
US Patent
Tarapeutics Science
US Patent
Affinity DataIC50: 103nMAssay Description:Enzymatic activity assay was conducted using EZH2(Y641F) TR-FRET assay KIT from Cisbio company on compounds that were shown to be active in primary s...More data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Homo sapiens (Human))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 110nMAssay Description:Inhibition of human iNOS assessed as reduction in L-[3H]-citrulline level using L-[3H]-arginine as substrate incubated for 1 hrMore data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2 [Y641F](Homo sapiens (Human))
Tarapeutics Science
US Patent
Tarapeutics Science
US Patent
Affinity DataIC50: 121nMAssay Description:Enzymatic activity assay was conducted using EZH2(Y641F) TR-FRET assay KIT from Cisbio company on compounds that were shown to be active in primary s...More data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Homo sapiens (Human))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 178nMAssay Description:Inhibition of human iNOS assessed as reduction in L-[3H]-citrulline level using L-[3H]-arginine as substrate incubated for 1 hrMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Homo sapiens (Human))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 188nMAssay Description:Inhibition of human iNOS assessed as reduction in L-[3H]-citrulline level using L-[3H]-arginine as substrate incubated for 1 hrMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Homo sapiens (Human))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 195nMAssay Description:Inhibition of human iNOS assessed as reduction in L-[3H]-citrulline level using L-[3H]-arginine as substrate incubated for 1 hrMore data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2 [Y641F](Homo sapiens (Human))
Tarapeutics Science
US Patent
Tarapeutics Science
US Patent
Affinity DataIC50: 219nMAssay Description:Enzymatic activity assay was conducted using EZH2(Y641F) TR-FRET assay KIT from Cisbio company on compounds that were shown to be active in primary s...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2 [Y641F](Homo sapiens (Human))
Tarapeutics Science
US Patent
Tarapeutics Science
US Patent
Affinity DataIC50: 220nMAssay Description:Enzymatic activity assay was conducted using EZH2(Y641F) TR-FRET assay KIT from Cisbio company on compounds that were shown to be active in primary s...More data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Homo sapiens (Human))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 291nMAssay Description:Inhibition of human iNOS assessed as reduction in L-[3H]-citrulline level using L-[3H]-arginine as substrate incubated for 1 hrMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Homo sapiens (Human))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 421nMAssay Description:Inhibition of human iNOS assessed as reduction in L-[3H]-citrulline level using L-[3H]-arginine as substrate incubated for 1 hrMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Homo sapiens (Human))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 434nMAssay Description:Inhibition of human iNOS assessed as reduction in L-[3H]-citrulline level using L-[3H]-arginine as substrate incubated for 1 hrMore data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2 [Y641F](Homo sapiens (Human))
Tarapeutics Science
US Patent
Tarapeutics Science
US Patent
Affinity DataIC50: 444nMAssay Description:Enzymatic activity assay was conducted using EZH2(Y641F) TR-FRET assay KIT from Cisbio company on compounds that were shown to be active in primary s...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2 [Y641F](Homo sapiens (Human))
Tarapeutics Science
US Patent
Tarapeutics Science
US Patent
Affinity DataIC50: 515nMAssay Description:Enzymatic activity assay was conducted using EZH2(Y641F) TR-FRET assay KIT from Cisbio company on compounds that were shown to be active in primary s...More data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Homo sapiens (Human))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 594nMAssay Description:Inhibition of human iNOS assessed as reduction in L-[3H]-citrulline level using L-[3H]-arginine as substrate incubated for 1 hrMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Homo sapiens (Human))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 630nMAssay Description:Inhibition of human iNOS assessed as reduction in L-[3H]-citrulline level using L-[3H]-arginine as substrate incubated for 1 hrMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Homo sapiens (Human))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 640nMAssay Description:Inhibition of human iNOS assessed as reduction in L-[3H]-citrulline level using L-[3H]-arginine as substrate incubated for 1 hrMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Homo sapiens (Human))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 645nMAssay Description:Inhibition of human iNOS assessed as reduction in L-[3H]-citrulline level using L-[3H]-arginine as substrate incubated for 1 hrMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Homo sapiens (Human))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 731nMAssay Description:Inhibition of human iNOS assessed as reduction in L-[3H]-citrulline level using L-[3H]-arginine as substrate incubated for 1 hrMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Homo sapiens (Human))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 765nMAssay Description:Inhibition of human iNOS assessed as reduction in L-[3H]-citrulline level using L-[3H]-arginine as substrate incubated for 1 hrMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Homo sapiens (Human))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 766nMAssay Description:Inhibition of human iNOS assessed as reduction in L-[3H]-citrulline level using L-[3H]-arginine as substrate incubated for 1 hrMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Homo sapiens (Human))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 782nMAssay Description:Inhibition of human iNOS assessed as reduction in L-[3H]-citrulline level using L-[3H]-arginine as substrate incubated for 1 hrMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Homo sapiens (Human))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 820nMAssay Description:Inhibition of human iNOS assessed as reduction in L-[3H]-citrulline level using L-[3H]-arginine as substrate incubated for 1 hrMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Homo sapiens (Human))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 892nMAssay Description:Inhibition of human iNOS assessed as reduction in L-[3H]-citrulline level using L-[3H]-arginine as substrate incubated for 1 hrMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Homo sapiens (Human))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 916nMAssay Description:Inhibition of human iNOS assessed as reduction in L-[3H]-citrulline level using L-[3H]-arginine as substrate incubated for 1 hrMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Homo sapiens (Human))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 945nMAssay Description:Inhibition of human iNOS assessed as reduction in L-[3H]-citrulline level using L-[3H]-arginine as substrate incubated for 1 hrMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Homo sapiens (Human))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 993nMAssay Description:Inhibition of human iNOS assessed as reduction in L-[3H]-citrulline level using L-[3H]-arginine as substrate incubated for 1 hrMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70E+3nMAssay Description:Disruption of His-tagged full length HSP90 (unknown origin)/GST-tagged Cdc37 (unknown origin) measured after 1 hr by HTRF methodMore data for this Ligand-Target Pair
Ligand Info
TargetNitric oxide synthase, brain(Homo sapiens (Human))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 1.98E+3nMAssay Description:Inhibition of human nNOS assessed as reduction in L-[3H]-citrulline level using L-[3H]-arginine as substrate incubated for 1 hrMore data for this Ligand-Target Pair
Affinity DataIC50: 2.40E+3nMAssay Description:Disruption of His-tagged full length HSP90 (unknown origin)/GST-tagged Cdc37 (unknown origin) measured after 1 hr by HTRF methodMore data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 2.90E+3nMAssay Description:Disruption of His-tagged full length HSP90 (unknown origin)/GST-tagged Cdc37 (unknown origin) measured after 1 hr by HTRF methodMore data for this Ligand-Target Pair
Ligand Info
TargetNitric oxide synthase, endothelial(Homo sapiens (Human))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 5.60E+3nMAssay Description:Inhibition of human eNOS assessed as reduction in L-[3H]-citrulline level using L-[3H]-arginine as substrate incubated for 1 hrMore data for this Ligand-Target Pair
TargetNitric oxide synthase, brain(Homo sapiens (Human))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 6.12E+3nMAssay Description:Inhibition of human nNOS assessed as reduction in L-[3H]-citrulline level using L-[3H]-arginine as substrate incubated for 1 hrMore data for this Ligand-Target Pair
Affinity DataIC50: 6.20E+3nMAssay Description:Disruption of His-tagged full length HSP90 (unknown origin)/GST-tagged Cdc37 (unknown origin) measured after 1 hr by HTRF methodMore data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 6.70E+3nMAssay Description:Disruption of His-tagged full length HSP90 (unknown origin)/GST-tagged Cdc37 (unknown origin) measured after 1 hr by HTRF methodMore data for this Ligand-Target Pair
Ligand Info
TargetNitric oxide synthase, brain(Homo sapiens (Human))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 8.36E+3nMAssay Description:Inhibition of human nNOS assessed as reduction in L-[3H]-citrulline level using L-[3H]-arginine as substrate incubated for 1 hrMore data for this Ligand-Target Pair
TargetNitric oxide synthase, endothelial(Homo sapiens (Human))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 9.60E+3nMAssay Description:Inhibition of human eNOS assessed as reduction in L-[3H]-citrulline level using L-[3H]-arginine as substrate incubated for 1 hrMore data for this Ligand-Target Pair





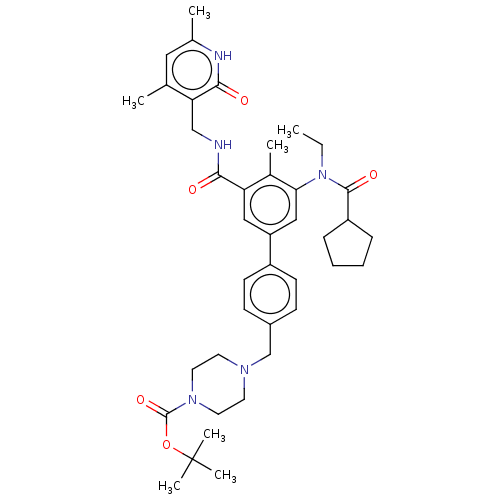
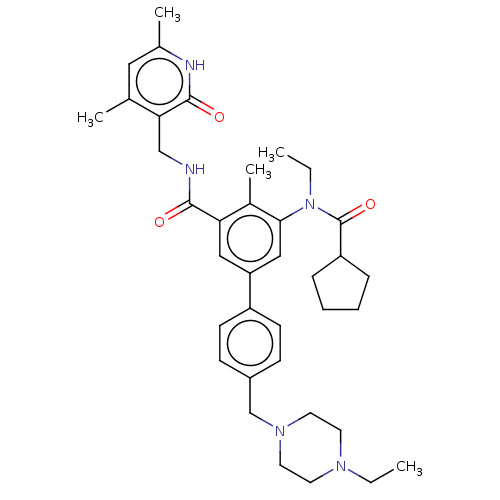

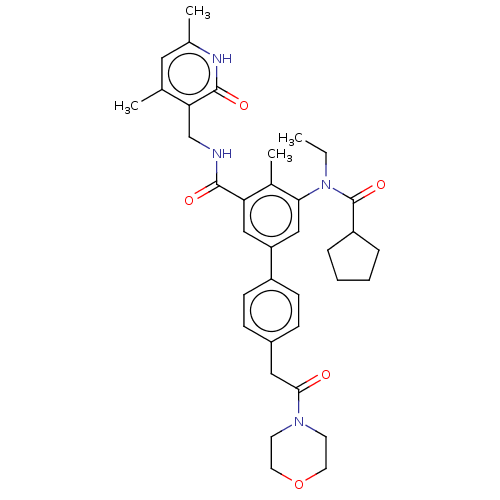
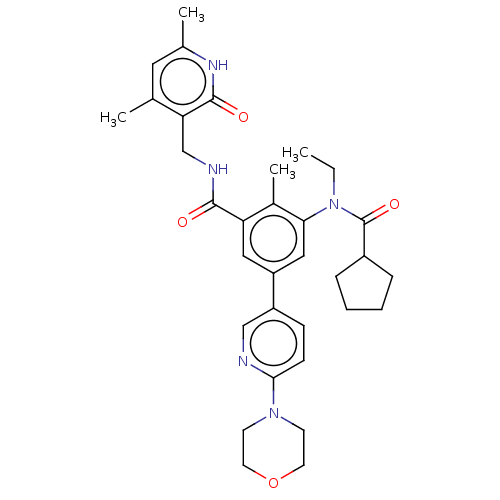





 3D Structure (crystal)
3D Structure (crystal)