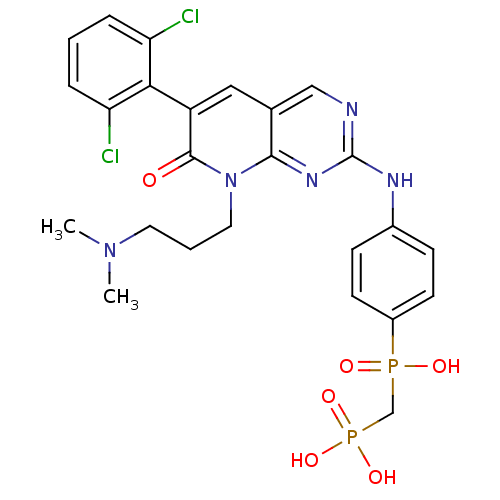
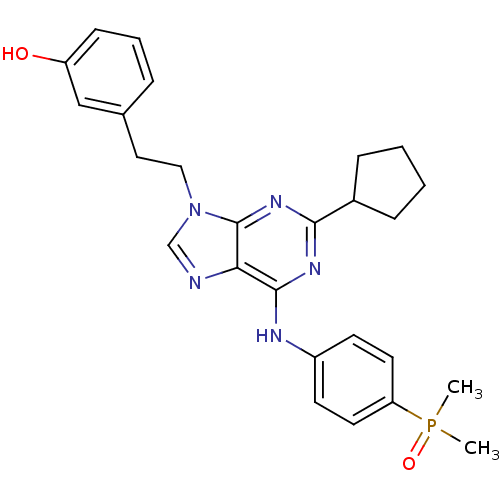
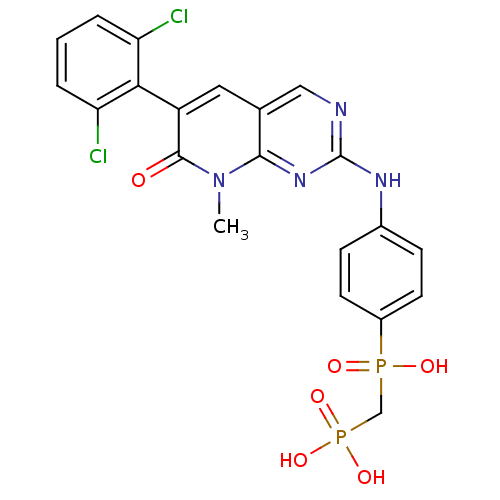
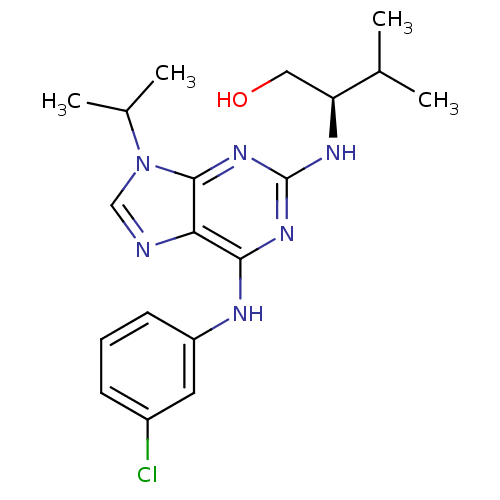
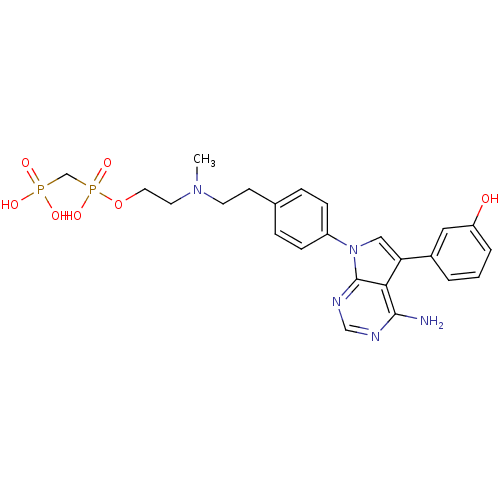
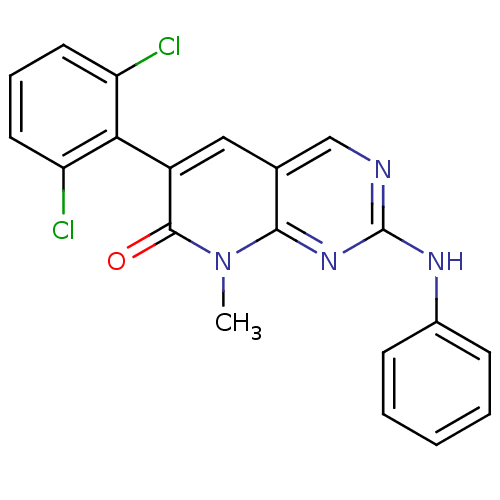
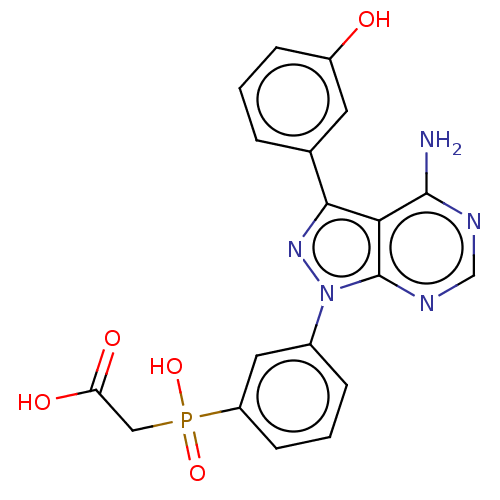
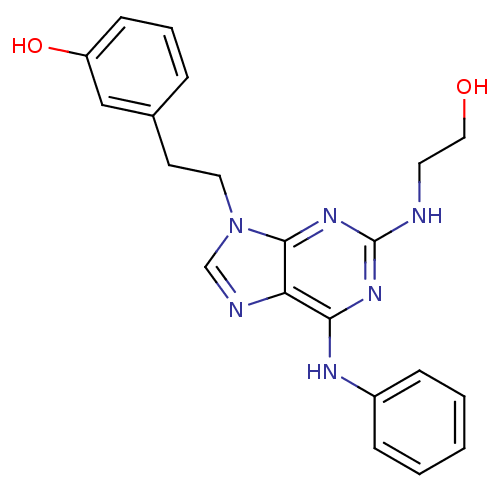
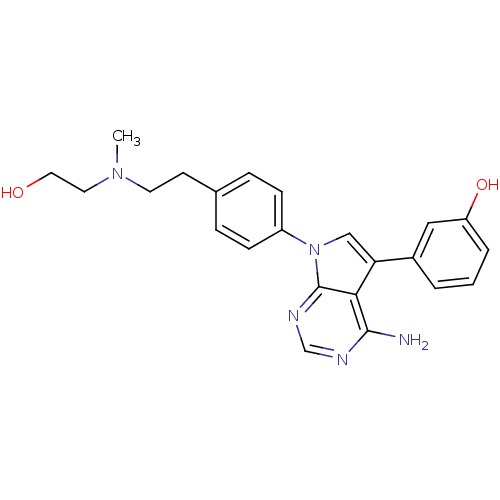
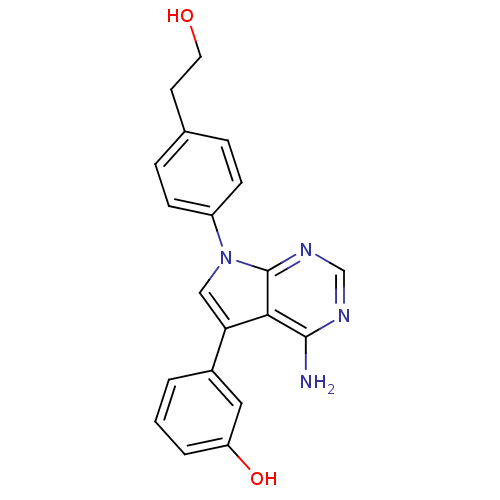
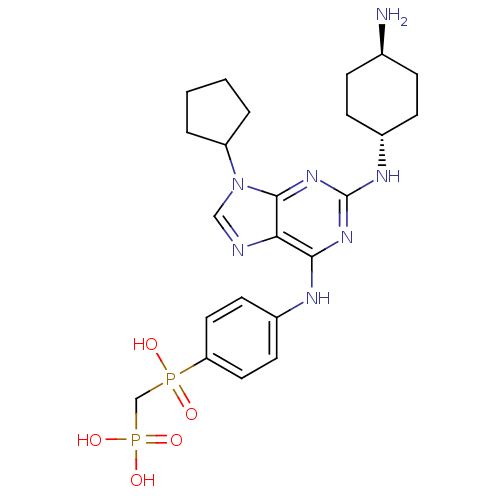
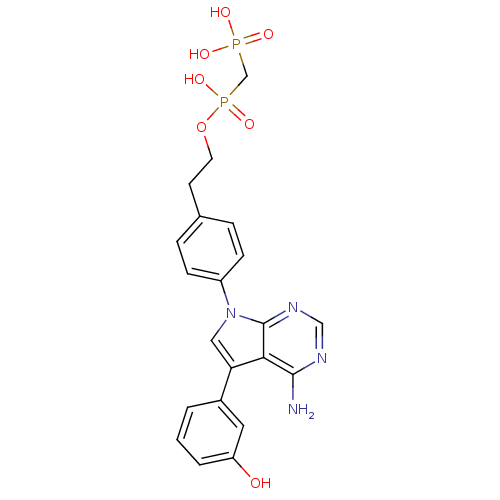
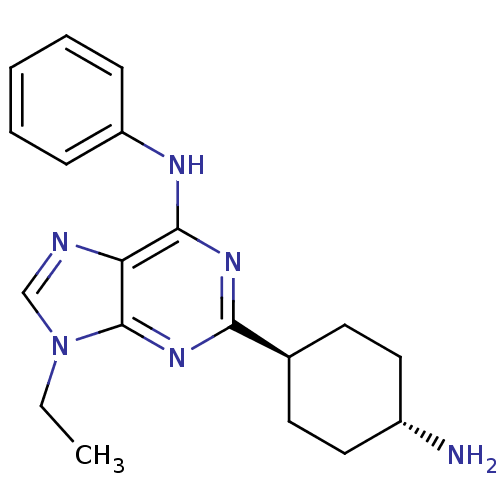
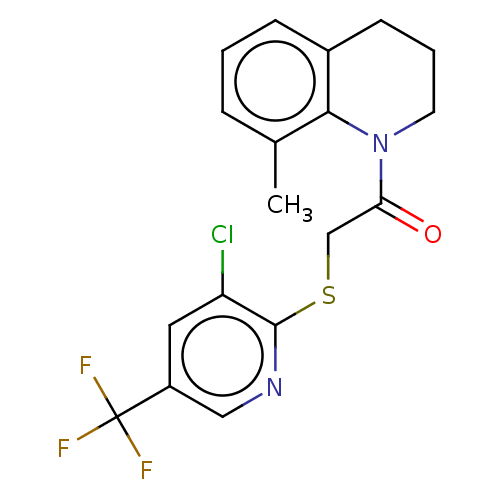
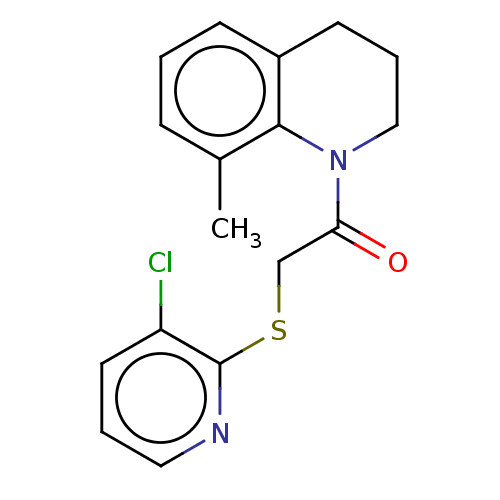
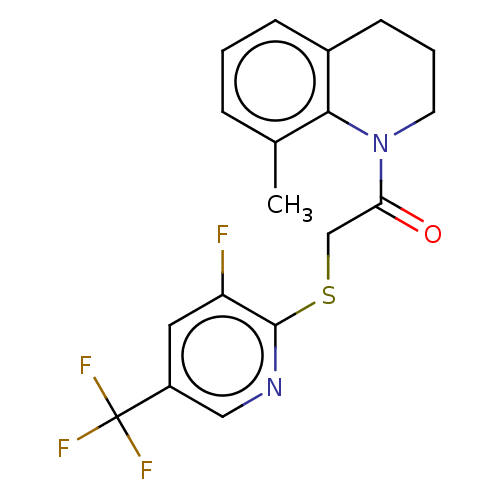
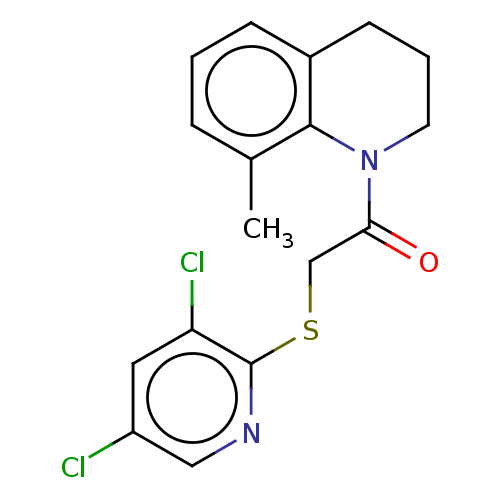
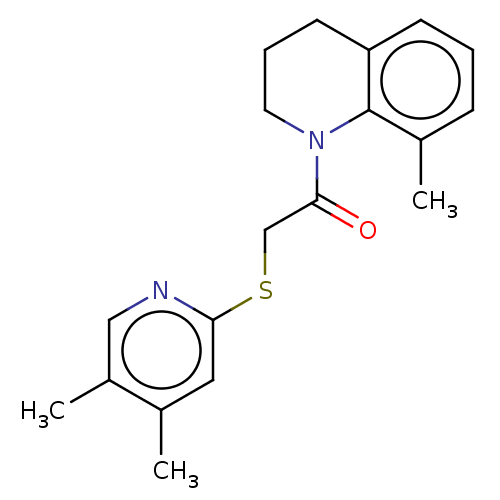
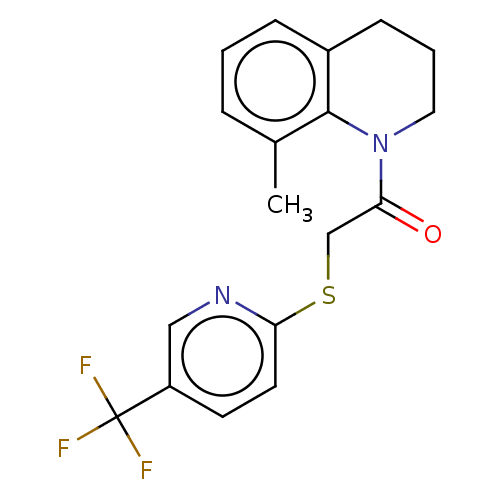
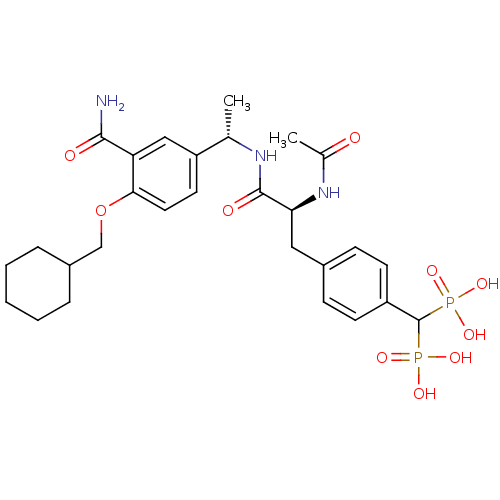
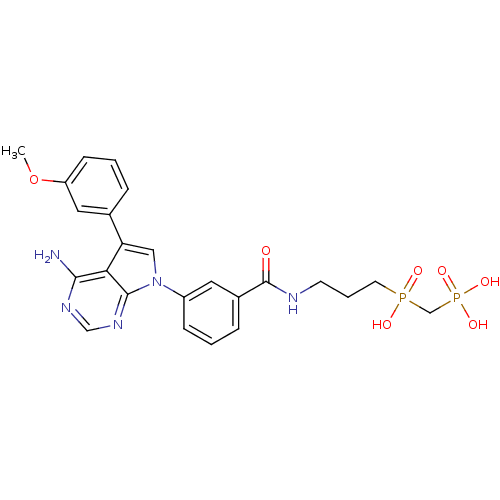
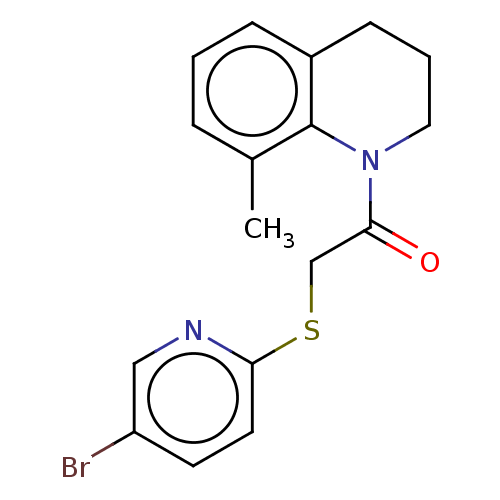
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Ariad Pharmaceuticals
Curated by ChEMBL
Ariad Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Inhibition of Src protein tryrosine kinaseMore data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Ariad Pharmaceuticals
Curated by ChEMBL
Ariad Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 0.450nMpH: 7.4 T: 2°CAssay Description:Inhibition of human Src kinase activity by ARIAD compounds were measured in a homogeneous time-resolved fluorescence resonance energy transfer (TR-FR...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Ariad Pharmaceuticals
Curated by ChEMBL
Ariad Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of Src protein tryrosine kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibition of Cyclin-dependent kinase 2 (CDK2)More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Ariad Pharmaceuticals
Curated by ChEMBL
Ariad Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:In vitro inhibition of Src protein tryrosine kinase using the Scintillation proximity kinase assay.More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Ariad Pharmaceuticals
Curated by ChEMBL
Ariad Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 16nMAssay Description:Inhibition of Src protein tryrosine kinaseMore data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Ariad Pharmaceuticals
Curated by ChEMBL
Ariad Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 20nMAssay Description:In vitro inhibition of Src protein tryrosine kinase using the Scintillation proximity kinase assay.More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Ariad Pharmaceuticals
Curated by ChEMBL
Ariad Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 25nMpH: 7.4 T: 2°CAssay Description:Inhibition of human Src kinase activity by ARIAD compounds were measured in a homogeneous time-resolved fluorescence resonance energy transfer (TR-FR...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Ariad Pharmaceuticals
Curated by ChEMBL
Ariad Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 26nMpH: 7.4 T: 2°CAssay Description:Inhibition of human Src kinase activity by ARIAD compounds were measured in a homogeneous time-resolved fluorescence resonance energy transfer (TR-FR...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Ariad Pharmaceuticals
Curated by ChEMBL
Ariad Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 34nMpH: 7.4 T: 2°CAssay Description:Inhibition of human Src kinase activity by ARIAD compounds were measured in a homogeneous time-resolved fluorescence resonance energy transfer (TR-FR...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Ariad Pharmaceuticals
Curated by ChEMBL
Ariad Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 40nMAssay Description:In vitro inhibition of Src protein tryrosine kinase using the Scintillation proximity kinase assay.More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Ariad Pharmaceuticals
Curated by ChEMBL
Ariad Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 40nMAssay Description:In vitro inhibition of Src protein tryrosine kinase using the Scintillation proximity kinase assay.More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Ariad Pharmaceuticals
Curated by ChEMBL
Ariad Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 41nMAssay Description:Inhibition of Src-mediated dentine resorption in rabbit-osteoclast assayMore data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Ariad Pharmaceuticals
Curated by ChEMBL
Ariad Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 60nMAssay Description:In vitro inhibition of Src protein tryrosine kinase using the Scintillation proximity kinase assay.More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Ariad Pharmaceuticals
Curated by ChEMBL
Ariad Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 67nMpH: 7.4 T: 2°CAssay Description:Inhibition of human Src kinase activity by ARIAD compounds were measured in a homogeneous time-resolved fluorescence resonance energy transfer (TR-FR...More data for this Ligand-Target Pair
Affinity DataIC50: 68nMAssay Description:2,6,9-Trisubstituted purines were evaluated for their src kinase inhibitory activities using an ELISA assay.More data for this Ligand-Target Pair
Affinity DataIC50: 70nMpH: 7.4 T: 2°CAssay Description:Inhibition of human Src kinase activity by ARIAD compounds were measured in a homogeneous time-resolved fluorescence resonance energy transfer (TR-FR...More data for this Ligand-Target Pair
Affinity DataIC50: 73nMAssay Description:2,6,9-Trisubstituted purines were evaluated for their src kinase inhibitory activities using an ELISA assay.More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Ariad Pharmaceuticals
Curated by ChEMBL
Ariad Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 77nMAssay Description:Inhibition of Src protein tryrosine kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 101nMAssay Description:2,6,9-Trisubstituted purines were evaluated for their src kinase inhibitory activities using an ELISA assay.More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Ariad Pharmaceuticals
Curated by ChEMBL
Ariad Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 120nMAssay Description:In vitro inhibition of Src protein tryrosine kinase using the Scintillation proximity kinase assay.More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Ariad Pharmaceuticals
Curated by ChEMBL
Ariad Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 141nMAssay Description:Inhibition of Src-mediated dentine resorption in rabbit-osteoclast assayMore data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Ariad Pharmaceuticals
Curated by ChEMBL
Ariad Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 160nMAssay Description:In vitro inhibition of Src protein tryrosine kinase using the Scintillation proximity kinase assay.More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 3(Homo sapiens (Human))
Hydra Biosciences
US Patent
Hydra Biosciences
US Patent
Affinity DataIC50: <200nMAssay Description:Phase 1: TRPV3 cells were induced 20-48 hours, removed from growth plates, and replated at low density (to attain good single-cell physical separatio...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 3(Homo sapiens (Human))
Hydra Biosciences
US Patent
Hydra Biosciences
US Patent
Affinity DataIC50: <200nMAssay Description:Phase 1: TRPV3 cells were induced 20-48 hours, removed from growth plates, and replated at low density (to attain good single-cell physical separatio...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Ariad Pharmaceuticals
Curated by ChEMBL
Ariad Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 200nMAssay Description:Inhibition of Src protein tryrosine kinaseMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 3(Homo sapiens (Human))
Hydra Biosciences
US Patent
Hydra Biosciences
US Patent
Affinity DataIC50: <200nMAssay Description:Phase 1: TRPV3 cells were induced 20-48 hours, removed from growth plates, and replated at low density (to attain good single-cell physical separatio...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 3(Homo sapiens (Human))
Hydra Biosciences
US Patent
Hydra Biosciences
US Patent
Affinity DataIC50: <200nMAssay Description:Phase 1: TRPV3 cells were induced 20-48 hours, removed from growth plates, and replated at low density (to attain good single-cell physical separatio...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 3(Homo sapiens (Human))
Hydra Biosciences
US Patent
Hydra Biosciences
US Patent
Affinity DataIC50: <200nMAssay Description:Phase 1: TRPV3 cells were induced 20-48 hours, removed from growth plates, and replated at low density (to attain good single-cell physical separatio...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 3(Homo sapiens (Human))
Hydra Biosciences
US Patent
Hydra Biosciences
US Patent
Affinity DataIC50: <200nMAssay Description:To determine whether compounds were selective for TRPV3 inhibition over inhibition of other ion channel types, the human ERG (hERG), NaV1.2, and TRPV...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 3(Homo sapiens (Human))
Hydra Biosciences
US Patent
Hydra Biosciences
US Patent
Affinity DataIC50: <200nMAssay Description:To determine whether compounds were selective for TRPV3 inhibition over inhibition of other ion channel types, the human ERG (hERG), NaV1.2, and TRPV...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 3(Homo sapiens (Human))
Hydra Biosciences
US Patent
Hydra Biosciences
US Patent
Affinity DataIC50: <200nMAssay Description:To determine whether compounds were selective for TRPV3 inhibition over inhibition of other ion channel types, the human ERG (hERG), NaV1.2, and TRPV...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 3(Homo sapiens (Human))
Hydra Biosciences
US Patent
Hydra Biosciences
US Patent
Affinity DataIC50: <200nMAssay Description:To determine whether compounds were selective for TRPV3 inhibition over inhibition of other ion channel types, the human ERG (hERG), NaV1.2, and TRPV...More data for this Ligand-Target Pair
Affinity DataIC50: 205nMAssay Description:2,6,9-Trisubstituted purines were evaluated for their src kinase inhibitory activities using an ELISA assay.More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Ariad Pharmaceuticals
Curated by ChEMBL
Ariad Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 239nMpH: 7.4 T: 2°CAssay Description:Inhibition of human Src kinase activity by ARIAD compounds were measured in a homogeneous time-resolved fluorescence resonance energy transfer (TR-FR...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Ariad Pharmaceuticals
Curated by ChEMBL
Ariad Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 240nMpH: 7.4 T: 2°CAssay Description:Inhibition of human Src kinase activity by ARIAD compounds were measured in a homogeneous time-resolved fluorescence resonance energy transfer (TR-FR...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Ariad Pharmaceuticals
Curated by ChEMBL
Ariad Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 250nMAssay Description:In vitro inhibition of Src protein tryrosine kinase using the Scintillation proximity kinase assay.More data for this Ligand-Target Pair
Affinity DataIC50: 273nMAssay Description:2,6,9-Trisubstituted purines were evaluated for their src kinase inhibitory activities using an ELISA assay.More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Ariad Pharmaceuticals
Curated by ChEMBL
Ariad Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 310nMAssay Description:In vitro inhibition of Src protein tryrosine kinase using the Scintillation proximity kinase assay.More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Ariad Pharmaceuticals
Curated by ChEMBL
Ariad Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 320nMAssay Description:In vitro inhibition of Src protein tryrosine kinase using the Scintillation proximity kinase assay.More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Ariad Pharmaceuticals
Curated by ChEMBL
Ariad Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 350nMAssay Description:Inhibition of binding to Src SH2 domainMore data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Ariad Pharmaceuticals
Curated by ChEMBL
Ariad Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 360nMAssay Description:In vitro inhibition of Src protein tryrosine kinase using the Scintillation proximity kinase assay.More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Ariad Pharmaceuticals
Curated by ChEMBL
Ariad Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 400nMAssay Description:In vitro inhibition of Src protein tryrosine kinase using the Scintillation proximity kinase assay.More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 3(Homo sapiens (Human))
Hydra Biosciences
US Patent
Hydra Biosciences
US Patent
Affinity DataIC50: <500nMAssay Description:To determine whether compounds were selective for TRPV3 inhibition over inhibition of other ion channel types, the human ERG (hERG), NaV1.2, and TRPV...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 3(Homo sapiens (Human))
Hydra Biosciences
US Patent
Hydra Biosciences
US Patent
Affinity DataIC50: <500nMAssay Description:To determine whether compounds were selective for TRPV3 inhibition over inhibition of other ion channel types, the human ERG (hERG), NaV1.2, and TRPV...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 3(Homo sapiens (Human))
Hydra Biosciences
US Patent
Hydra Biosciences
US Patent
Affinity DataIC50: <500nMAssay Description:Phase 1: TRPV3 cells were induced 20-48 hours, removed from growth plates, and replated at low density (to attain good single-cell physical separatio...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 3(Homo sapiens (Human))
Hydra Biosciences
US Patent
Hydra Biosciences
US Patent
Affinity DataIC50: <500nMAssay Description:Phase 1: TRPV3 cells were induced 20-48 hours, removed from growth plates, and replated at low density (to attain good single-cell physical separatio...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 3(Homo sapiens (Human))
Hydra Biosciences
US Patent
Hydra Biosciences
US Patent
Affinity DataIC50: <500nMAssay Description:To determine whether compounds were selective for TRPV3 inhibition over inhibition of other ion channel types, the human ERG (hERG), NaV1.2, and TRPV...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 3(Homo sapiens (Human))
Hydra Biosciences
US Patent
Hydra Biosciences
US Patent
Affinity DataIC50: <500nMAssay Description:Phase 1: TRPV3 cells were induced 20-48 hours, removed from growth plates, and replated at low density (to attain good single-cell physical separatio...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 3(Homo sapiens (Human))
Hydra Biosciences
US Patent
Hydra Biosciences
US Patent
Affinity DataIC50: <500nMAssay Description:To determine whether compounds were selective for TRPV3 inhibition over inhibition of other ion channel types, the human ERG (hERG), NaV1.2, and TRPV...More data for this Ligand-Target Pair