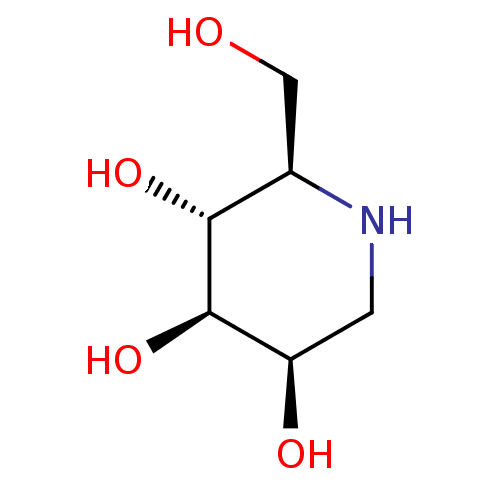
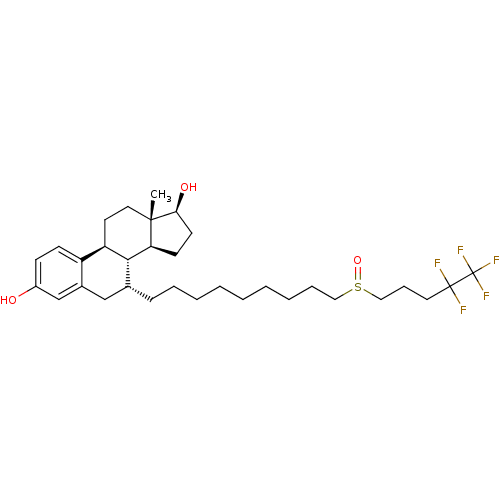
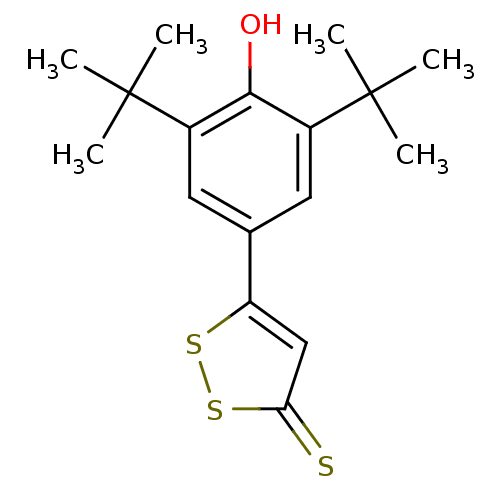
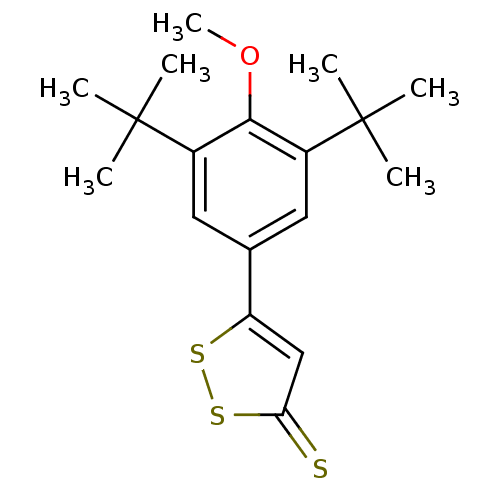
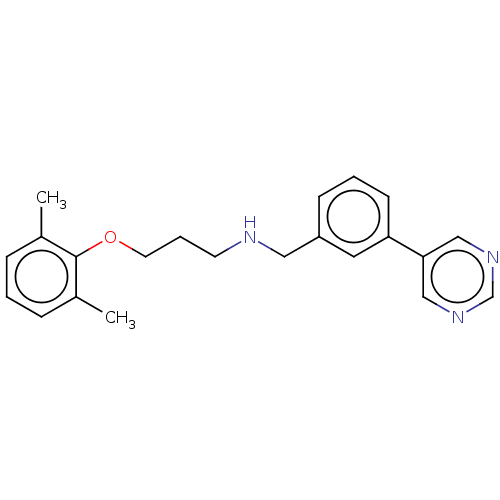
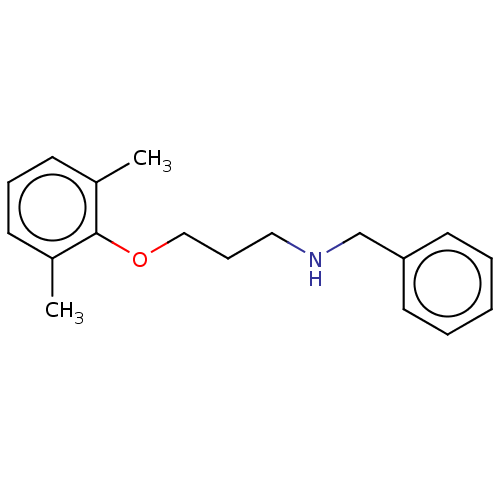
Affinity DataKi: 130nMAssay Description:Inhibition of Pseudomonas aeruginosa recombinant arylsulfatase A expressed in Escherichia coliMore data for this Ligand-Target Pair
TargetAlpha-1,2-mannosidase, putative(Bacteroides thetaiotaomicron )
Newcastle University
Curated by ChEMBL
Newcastle University
Curated by ChEMBL
Affinity DataKi: 400nMAssay Description:Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 3965 assessed as reduction of mannose release using 4NP-mannopyranoside substrateMore data for this Ligand-Target Pair
TargetAlpha-1,2-mannosidase, putative(Bacteroides thetaiotaomicron )
Newcastle University
Curated by ChEMBL
Newcastle University
Curated by ChEMBL
Affinity DataKi: 1.00E+3nMAssay Description:Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 3130 assessed as reduction of mannose release using 4NP-mannopyranoside substrateMore data for this Ligand-Target Pair
TargetAlpha-1,2-mannosidase, putative(Bacteroides thetaiotaomicron )
Newcastle University
Curated by ChEMBL
Newcastle University
Curated by ChEMBL
Affinity DataKi: 5.00E+3nMAssay Description:Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 3990 assessed as reduction of mannose release using 4NP-mannopyranoside substrateMore data for this Ligand-Target Pair
TargetAlpha-1,2-mannosidase, putative(Bacteroides thetaiotaomicron )
Newcastle University
Curated by ChEMBL
Newcastle University
Curated by ChEMBL
Affinity DataKi: 1.40E+4nMAssay Description:Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 2199 assessed as reduction of mannose release using 4NP-mannopyranoside substrateMore data for this Ligand-Target Pair
TargetAlpha-1,2-mannosidase, putative(Bacteroides thetaiotaomicron )
Newcastle University
Curated by ChEMBL
Newcastle University
Curated by ChEMBL
Affinity DataKi: 4.30E+4nMAssay Description:Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 3994 assessed as reduction of mannose release using 4NP-mannopyranoside substrateMore data for this Ligand-Target Pair
TargetAlpha-1,2-mannosidase, putative(Bacteroides thetaiotaomicron )
Newcastle University
Curated by ChEMBL
Newcastle University
Curated by ChEMBL
Affinity DataKi: 4.60E+4nMAssay Description:Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 3991 assessed as reduction of mannose release using 4NP-mannopyranoside substrateMore data for this Ligand-Target Pair
TargetAlpha-1,2-mannosidase, putative(Bacteroides thetaiotaomicron )
Newcastle University
Curated by ChEMBL
Newcastle University
Curated by ChEMBL
Affinity DataKi: 7.00E+4nMAssay Description:Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 2948 assessed as reduction of mannose release using 4NP-mannopyranoside substrateMore data for this Ligand-Target Pair
TargetAlpha-1,2-mannosidase, putative(Bacteroides thetaiotaomicron )
Newcastle University
Curated by ChEMBL
Newcastle University
Curated by ChEMBL
Affinity DataKi: 9.00E+4nMAssay Description:Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 2199 assessed as reduction of mannose release using 4NP-mannopyranoside substrateMore data for this Ligand-Target Pair
TargetAlpha-1,2-mannosidase, putative(Bacteroides thetaiotaomicron )
Newcastle University
Curated by ChEMBL
Newcastle University
Curated by ChEMBL
Affinity DataKi: 9.60E+4nMAssay Description:Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 3990 assessed as reduction of mannose release using 4NP-mannopyranoside substrateMore data for this Ligand-Target Pair
TargetAlpha-1,2-mannosidase, putative(Bacteroides thetaiotaomicron )
Newcastle University
Curated by ChEMBL
Newcastle University
Curated by ChEMBL
Affinity DataKi: 1.40E+5nMAssay Description:Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 3990 assessed as reduction of mannose release using 4NP-mannopyranoside substrateMore data for this Ligand-Target Pair
TargetAlpha-1,2-mannosidase, putative(Bacteroides thetaiotaomicron )
Newcastle University
Curated by ChEMBL
Newcastle University
Curated by ChEMBL
Affinity DataKi: 2.30E+5nMAssay Description:Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 2199 assessed as reduction of mannose release using 4NP-mannopyranoside substrateMore data for this Ligand-Target Pair
TargetAlpha-1,2-mannosidase, putative(Bacteroides thetaiotaomicron )
Newcastle University
Curated by ChEMBL
Newcastle University
Curated by ChEMBL
Affinity DataKi: 2.60E+5nMAssay Description:Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 3858 assessed as reduction of mannose release using 4NP-mannopyranoside substrateMore data for this Ligand-Target Pair
TargetAlpha-1,2-mannosidase, putative(Bacteroides thetaiotaomicron )
Newcastle University
Curated by ChEMBL
Newcastle University
Curated by ChEMBL
Affinity DataKi: 2.70E+5nMAssay Description:Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 3962 assessed as reduction of mannose release using 4NP-mannopyranoside substrateMore data for this Ligand-Target Pair
TargetAlpha-1,2-mannosidase, putative(Bacteroides thetaiotaomicron )
Newcastle University
Curated by ChEMBL
Newcastle University
Curated by ChEMBL
Affinity DataKi: 3.60E+5nMAssay Description:Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 4073 assessed as reduction of mannose release using 4NP-mannopyranoside substrateMore data for this Ligand-Target Pair
TargetAlpha-1,2-mannosidase/Alpha-1,2-mannosidase family protein/Alpha-1,2-mannosidase, putative/Glycoside hydrolase family 92/Putative alpha-1,2-mannosidase(Bacteroides thetaiotaomicron )
Newcastle University
Curated by ChEMBL
Newcastle University
Curated by ChEMBL
Affinity DataKi: 6.00E+5nMAssay Description:Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 1032 assessed as reduction of mannose release using 4NP-mannopyranoside substrateMore data for this Ligand-Target Pair
TargetAlpha-1,2-mannosidase, putative(Bacteroides thetaiotaomicron )
Newcastle University
Curated by ChEMBL
Newcastle University
Curated by ChEMBL
Affinity DataKi: 7.90E+5nMAssay Description:Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 3963 assessed as reduction of mannose release using 4NP-mannopyranoside substrateMore data for this Ligand-Target Pair
TargetAlpha-1,2-mannosidase, putative(Bacteroides thetaiotaomicron )
Newcastle University
Curated by ChEMBL
Newcastle University
Curated by ChEMBL
Affinity DataKi: 8.60E+5nMAssay Description:Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 4093 assessed as reduction of mannose release using 4NP-mannopyranoside substrateMore data for this Ligand-Target Pair
TargetAlpha-1,2-mannosidase, putative(Bacteroides thetaiotaomicron )
Newcastle University
Curated by ChEMBL
Newcastle University
Curated by ChEMBL
Affinity DataKi: 1.60E+6nMAssay Description:Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 4092 assessed as reduction of mannose release using 4NP-mannopyranoside substrateMore data for this Ligand-Target Pair
TargetAlpha-1,2-mannosidase, putative(Bacteroides thetaiotaomicron )
Newcastle University
Curated by ChEMBL
Newcastle University
Curated by ChEMBL
Affinity DataKi: 1.80E+6nMAssay Description:Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 1878 assessed as reduction of mannose release using 4NP-mannopyranoside substrateMore data for this Ligand-Target Pair
TargetAlpha-1,2-mannosidase, putative(Bacteroides thetaiotaomicron )
Newcastle University
Curated by ChEMBL
Newcastle University
Curated by ChEMBL
Affinity DataKi: 5.80E+6nMAssay Description:Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 3784 assessed as reduction of mannose release using 4NP-mannopyranoside substrateMore data for this Ligand-Target Pair
TargetAlpha-1,2-mannosidase, putative(Bacteroides thetaiotaomicron )
Newcastle University
Curated by ChEMBL
Newcastle University
Curated by ChEMBL
Affinity DataKi: 1.20E+7nMAssay Description:Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 3990 assessed as reduction of mannose release using 4NP-mannopyranoside substrateMore data for this Ligand-Target Pair
TargetAlpha-1,2-mannosidase, putative(Bacteroides thetaiotaomicron )
Newcastle University
Curated by ChEMBL
Newcastle University
Curated by ChEMBL
Affinity DataKi: 1.30E+7nMAssay Description:Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 2199 assessed as reduction of mannose release using 4NP-mannopyranoside substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 5.89nMAssay Description:Displacement of [3H]estradiol from rat uterine cytosolic estrogen receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 7.20nMAssay Description:Inhibition of human recombinant COX2 expressed in Sf21 cells assessed as effect on prostaglandin E2 production by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 28nMAssay Description:Inhibition of human platelet COX1 assessed as effect on prostaglandin E2 production by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 47nMAssay Description:Inhibition of human recombinant COX2 expressed in Sf21 cells assessed as effect on prostaglandin E2 production by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 75nMAssay Description:Inhibition of human recombinant COX2 expressed in Sf21 cells assessed as effect on prostaglandin E2 production by ELISAMore data for this Ligand-Target Pair
TargetSodium channel protein type 8 subunit alpha(Homo sapiens (Human))
The Florey Institute
US Patent
The Florey Institute
US Patent
Affinity DataIC50: 100nMAssay Description:Test Protocol for Determining the Potency of Compounds on Nav1.2 and Nav1.6 Subtypes of Voltage-Gated Sodium Ion Channels Expressed in Mammalian Cell...More data for this Ligand-Target Pair
TargetSodium channel protein type 8 subunit alpha(Homo sapiens (Human))
The Florey Institute
US Patent
The Florey Institute
US Patent
Affinity DataIC50: 100nMAssay Description:Test Protocol for Determining the Potency of Compounds on Nav1.2 and Nav1.6 Subtypes of Voltage-Gated Sodium Ion Channels Expressed in Mammalian Cell...More data for this Ligand-Target Pair
TargetSodium channel protein type 2 subunit alpha(Homo sapiens (Human))
The Florey Institute
US Patent
The Florey Institute
US Patent
Affinity DataIC50: 100nMAssay Description:Test Protocol for Determining the Potency of Compounds on Nav1.2 and Nav1.6 Subtypes of Voltage-Gated Sodium Ion Channels Expressed in Mammalian Cell...More data for this Ligand-Target Pair
TargetSodium channel protein type 2 subunit alpha(Homo sapiens (Human))
The Florey Institute
US Patent
The Florey Institute
US Patent
Affinity DataIC50: 100nMAssay Description:Test Protocol for Determining the Potency of Compounds on Nav1.2 and Nav1.6 Subtypes of Voltage-Gated Sodium Ion Channels Expressed in Mammalian Cell...More data for this Ligand-Target Pair
TargetSodium channel protein type 2 subunit alpha(Homo sapiens (Human))
The Florey Institute
US Patent
The Florey Institute
US Patent
Affinity DataIC50: 200nMAssay Description:Test Protocol for Determining the Potency of Compounds on Nav1.2 and Nav1.6 Subtypes of Voltage-Gated Sodium Ion Channels Expressed in Mammalian Cell...More data for this Ligand-Target Pair
TargetSodium channel protein type 2 subunit alpha(Homo sapiens (Human))
The Florey Institute
US Patent
The Florey Institute
US Patent
Affinity DataIC50: 200nMAssay Description:Test Protocol for Determining the Potency of Compounds on Nav1.2 and Nav1.6 Subtypes of Voltage-Gated Sodium Ion Channels Expressed in Mammalian Cell...More data for this Ligand-Target Pair
TargetSodium channel protein type 2 subunit alpha(Homo sapiens (Human))
The Florey Institute
US Patent
The Florey Institute
US Patent
Affinity DataIC50: 200nMAssay Description:Test Protocol for Determining the Potency of Compounds on Nav1.2 and Nav1.6 Subtypes of Voltage-Gated Sodium Ion Channels Expressed in Mammalian Cell...More data for this Ligand-Target Pair
TargetSodium channel protein type 2 subunit alpha(Homo sapiens (Human))
The Florey Institute
US Patent
The Florey Institute
US Patent
Affinity DataIC50: 200nMAssay Description:Test Protocol for Determining the Potency of Compounds on Nav1.2 and Nav1.6 Subtypes of Voltage-Gated Sodium Ion Channels Expressed in Mammalian Cell...More data for this Ligand-Target Pair
Affinity DataIC50: 234nMAssay Description:Displacement of [3H]estradiol from rat uterine cytosolic estrogen receptorMore data for this Ligand-Target Pair
TargetSodium channel protein type 8 subunit alpha(Homo sapiens (Human))
The Florey Institute
US Patent
The Florey Institute
US Patent
Affinity DataIC50: 300nMAssay Description:Test Protocol for Determining the Potency of Compounds on Nav1.2 and Nav1.6 Subtypes of Voltage-Gated Sodium Ion Channels Expressed in Mammalian Cell...More data for this Ligand-Target Pair
TargetSodium channel protein type 8 subunit alpha(Homo sapiens (Human))
The Florey Institute
US Patent
The Florey Institute
US Patent
Affinity DataIC50: 300nMAssay Description:Test Protocol for Determining the Potency of Compounds on Nav1.2 and Nav1.6 Subtypes of Voltage-Gated Sodium Ion Channels Expressed in Mammalian Cell...More data for this Ligand-Target Pair
TargetSodium channel protein type 8 subunit alpha(Homo sapiens (Human))
The Florey Institute
US Patent
The Florey Institute
US Patent
Affinity DataIC50: 300nMAssay Description:Test Protocol for Determining the Potency of Compounds on Nav1.2 and Nav1.6 Subtypes of Voltage-Gated Sodium Ion Channels Expressed in Mammalian Cell...More data for this Ligand-Target Pair
TargetSodium channel protein type 8 subunit alpha(Homo sapiens (Human))
The Florey Institute
US Patent
The Florey Institute
US Patent
Affinity DataIC50: 300nMAssay Description:Test Protocol for Determining the Potency of Compounds on Nav1.2 and Nav1.6 Subtypes of Voltage-Gated Sodium Ion Channels Expressed in Mammalian Cell...More data for this Ligand-Target Pair
TargetSodium channel protein type 8 subunit alpha(Homo sapiens (Human))
The Florey Institute
US Patent
The Florey Institute
US Patent
Affinity DataIC50: 400nMAssay Description:Test Protocol for Determining the Potency of Compounds on Nav1.2 and Nav1.6 Subtypes of Voltage-Gated Sodium Ion Channels Expressed in Mammalian Cell...More data for this Ligand-Target Pair
TargetSodium channel protein type 2 subunit alpha(Homo sapiens (Human))
The Florey Institute
US Patent
The Florey Institute
US Patent
Affinity DataIC50: 400nMAssay Description:Test Protocol for Determining the Potency of Compounds on Nav1.2 and Nav1.6 Subtypes of Voltage-Gated Sodium Ion Channels Expressed in Mammalian Cell...More data for this Ligand-Target Pair
TargetSodium channel protein type 2 subunit alpha(Homo sapiens (Human))
The Florey Institute
US Patent
The Florey Institute
US Patent
Affinity DataIC50: 400nMAssay Description:Test Protocol for Determining the Potency of Compounds on Nav1.2 and Nav1.6 Subtypes of Voltage-Gated Sodium Ion Channels Expressed in Mammalian Cell...More data for this Ligand-Target Pair
TargetSodium channel protein type 2 subunit alpha(Homo sapiens (Human))
The Florey Institute
US Patent
The Florey Institute
US Patent
Affinity DataIC50: 400nMAssay Description:Test Protocol for Determining the Potency of Compounds on Nav1.2 and Nav1.6 Subtypes of Voltage-Gated Sodium Ion Channels Expressed in Mammalian Cell...More data for this Ligand-Target Pair
TargetSodium channel protein type 2 subunit alpha(Homo sapiens (Human))
The Florey Institute
US Patent
The Florey Institute
US Patent
Affinity DataIC50: 400nMAssay Description:Test Protocol for Determining the Potency of Compounds on Nav1.2 and Nav1.6 Subtypes of Voltage-Gated Sodium Ion Channels Expressed in Mammalian Cell...More data for this Ligand-Target Pair
TargetSodium channel protein type 2 subunit alpha(Homo sapiens (Human))
The Florey Institute
US Patent
The Florey Institute
US Patent
Affinity DataIC50: 500nMAssay Description:Test Protocol for Determining the Potency of Compounds on Nav1.2 and Nav1.6 Subtypes of Voltage-Gated Sodium Ion Channels Expressed in Mammalian Cell...More data for this Ligand-Target Pair
TargetSodium channel protein type 2 subunit alpha(Homo sapiens (Human))
The Florey Institute
US Patent
The Florey Institute
US Patent
Affinity DataIC50: 500nMAssay Description:Test Protocol for Determining the Potency of Compounds on Nav1.2 and Nav1.6 Subtypes of Voltage-Gated Sodium Ion Channels Expressed in Mammalian Cell...More data for this Ligand-Target Pair
Affinity DataIC50: 661nMAssay Description:Displacement of [3H]estradiol from rat uterine cytosolic estrogen receptorMore data for this Ligand-Target Pair
TargetSodium channel protein type 8 subunit alpha(Homo sapiens (Human))
The Florey Institute
US Patent
The Florey Institute
US Patent
Affinity DataIC50: 800nMAssay Description:Test Protocol for Determining the Potency of Compounds on Nav1.2 and Nav1.6 Subtypes of Voltage-Gated Sodium Ion Channels Expressed in Mammalian Cell...More data for this Ligand-Target Pair


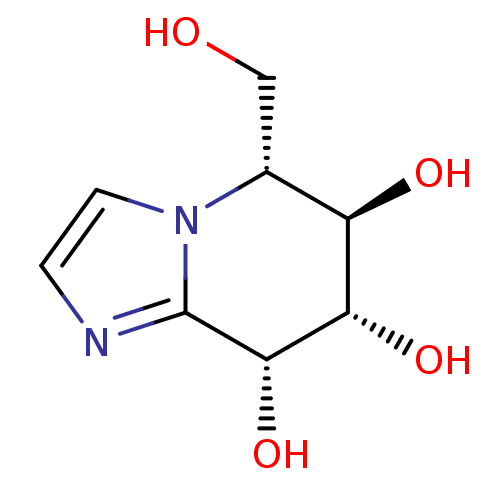
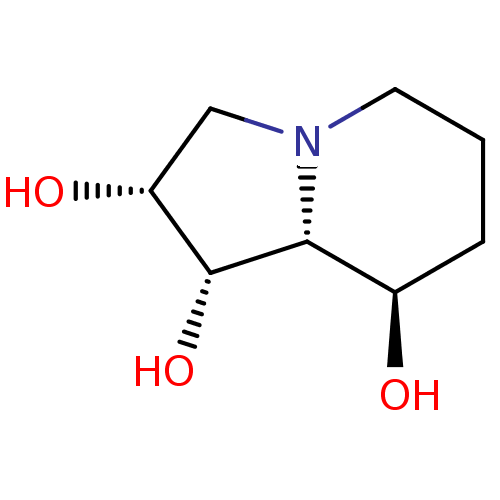
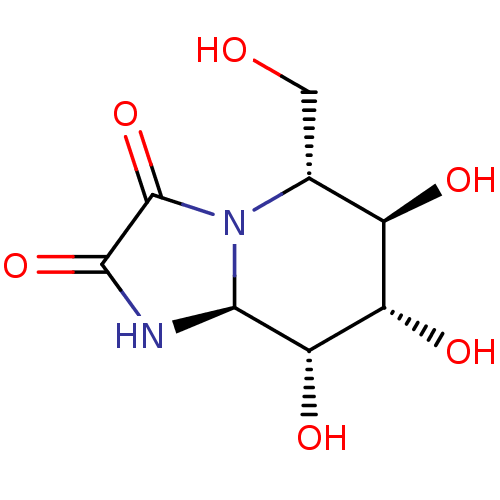
 3D Structure (crystal)
3D Structure (crystal)