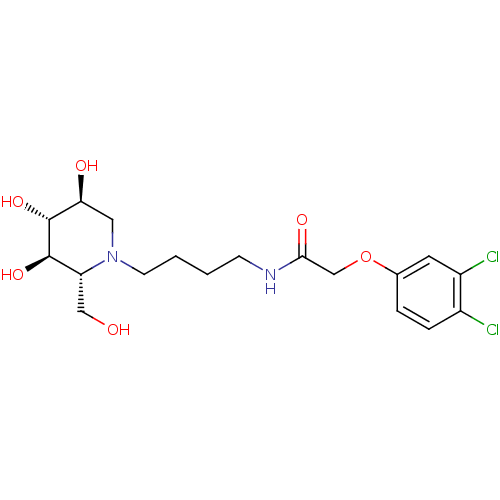
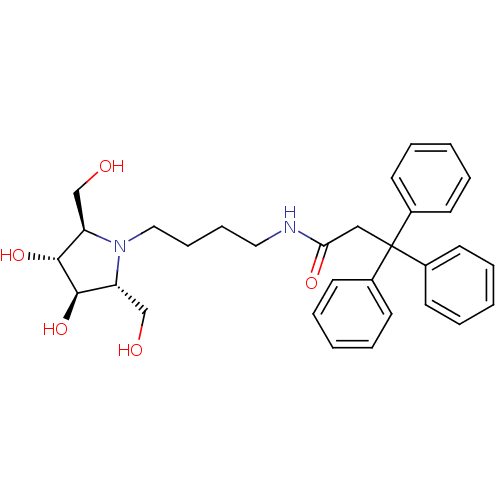
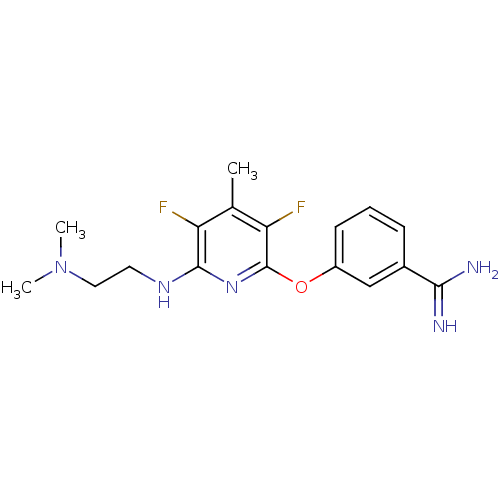
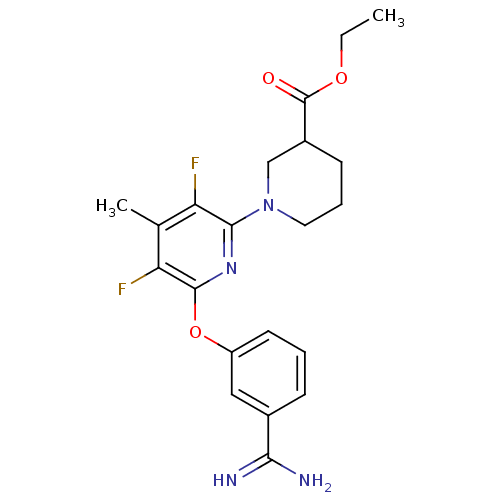
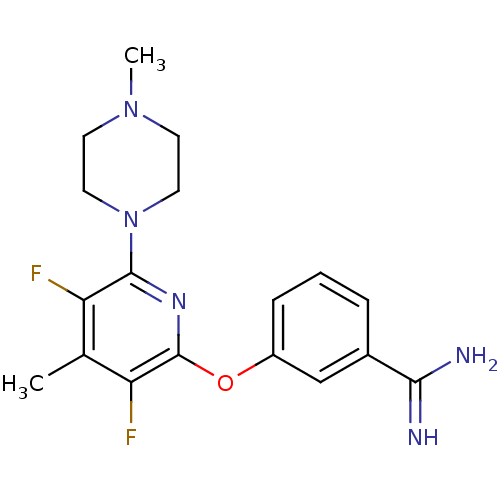
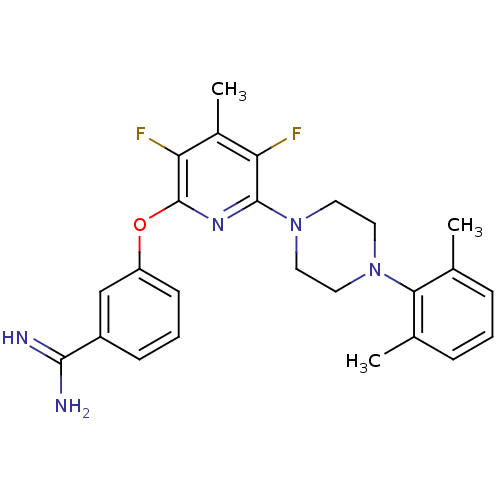
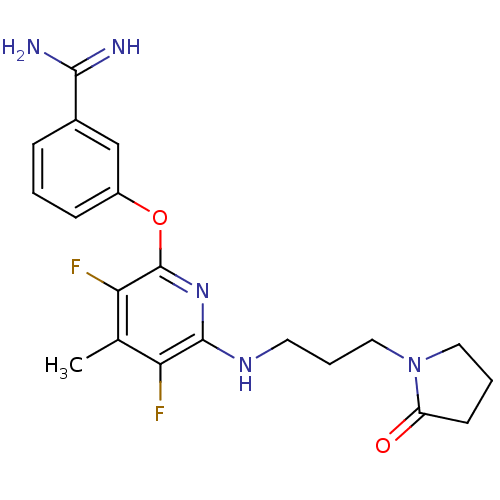
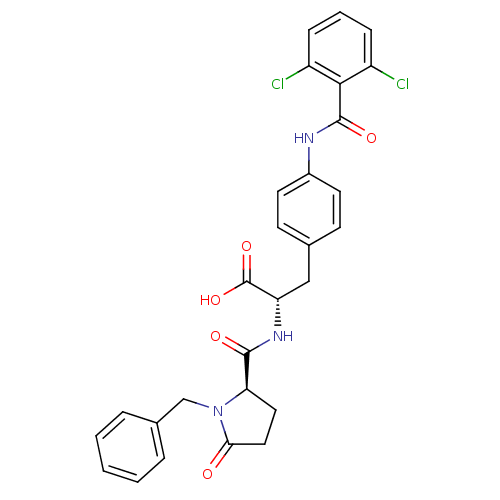
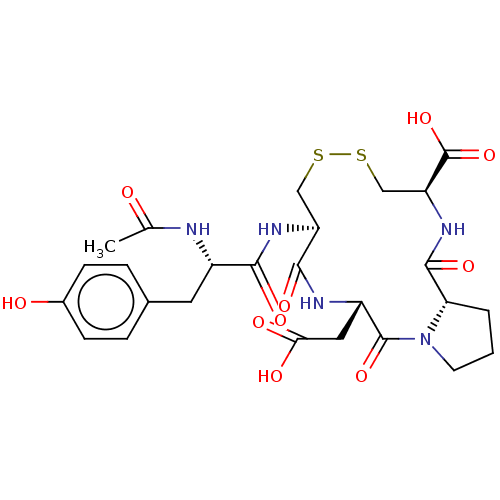
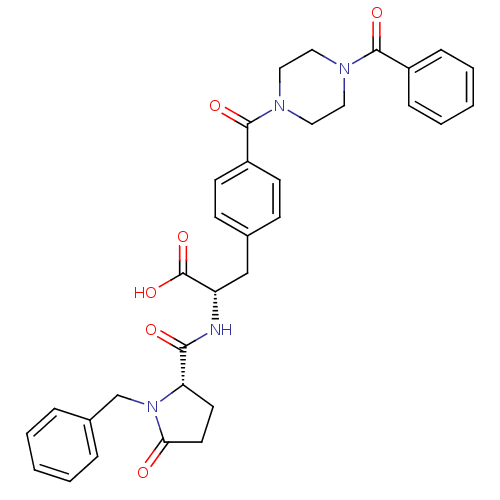
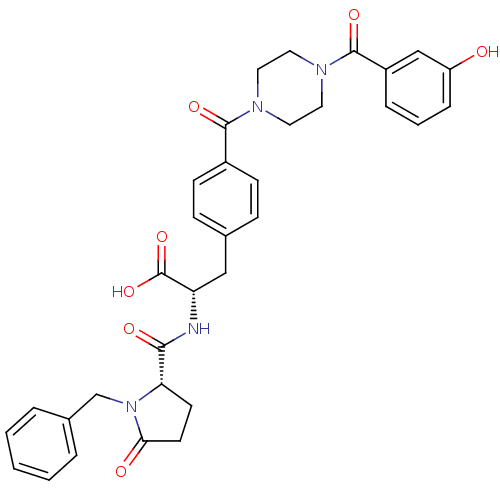
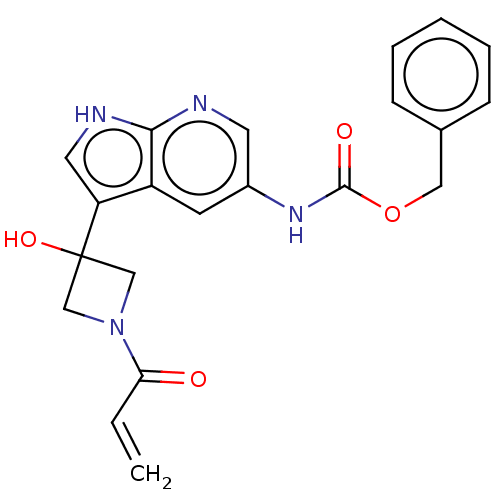
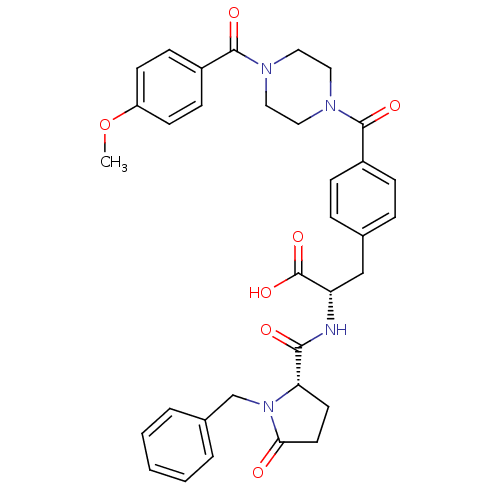
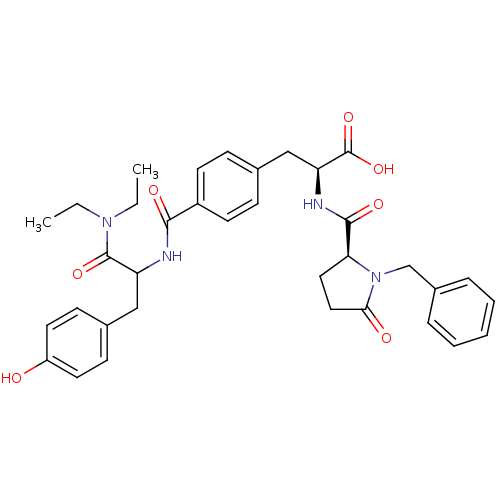
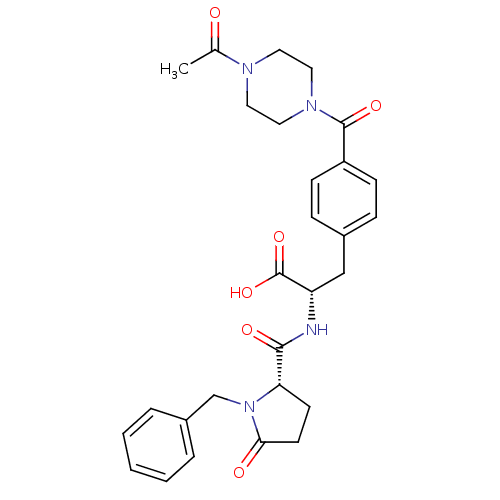
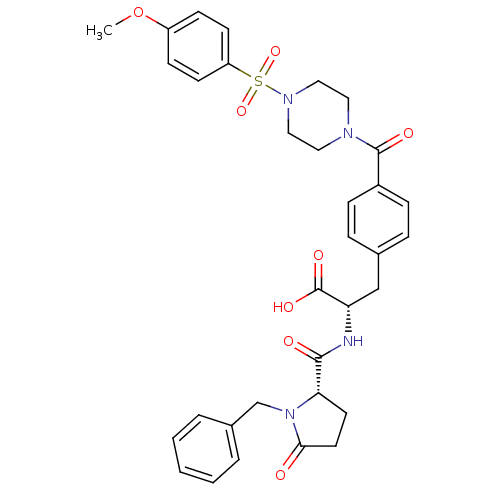
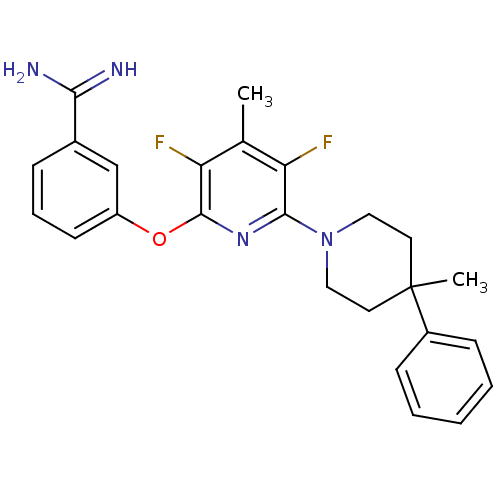
Affinity DataKi: 6.80nMAssay Description:Binding affinity of the compound was evaluated against Coagulation factor XMore data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:Binding affinity of the compound was evaluated against Coagulation factor XMore data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:Binding affinity of the compound was evaluated against Coagulation factor XMore data for this Ligand-Target Pair
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Genomics Research Center
Curated by ChEMBL
Genomics Research Center
Curated by ChEMBL
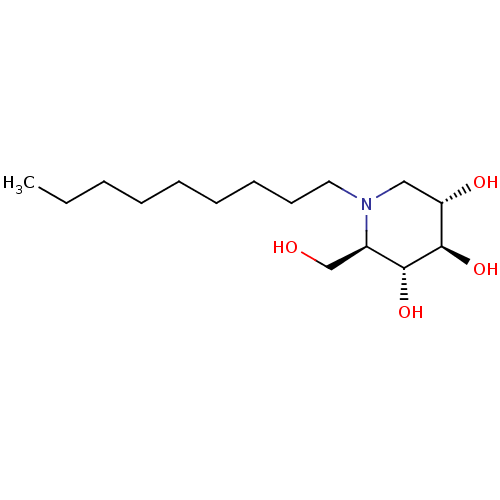
Affinity DataKi: 71nMAssay Description:Competitive inhibition of human beta-glucocerebrosidase by Lineweaver-Burk double reciprocal plot methodMore data for this Ligand-Target Pair
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Genomics Research Center
Curated by ChEMBL
Genomics Research Center
Curated by ChEMBL
Affinity DataKi: 400nMAssay Description:Competitive inhibition of human beta-glucocerebrosidase by Lineweaver-Burk double reciprocal plot methodMore data for this Ligand-Target Pair
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Genomics Research Center
Curated by ChEMBL
Genomics Research Center
Curated by ChEMBL
Affinity DataKi: 400nMAssay Description:Competitive inhibition of human beta-glucocerebrosidase by Lineweaver-Burk double reciprocal plot methodMore data for this Ligand-Target Pair
Affinity DataKi: 410nMAssay Description:Binding affinity of the compound was evaluated against Coagulation factor XMore data for this Ligand-Target Pair
Affinity DataKi: 495nMAssay Description:Binding affinity of the compound was evaluated against Coagulation factor XMore data for this Ligand-Target Pair
Affinity DataKi: 560nMAssay Description:Binding affinity of the compound was evaluated against Coagulation factor XMore data for this Ligand-Target Pair
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Genomics Research Center
Curated by ChEMBL
Genomics Research Center
Curated by ChEMBL
Affinity DataKi: 1.00E+3nMAssay Description:Competitive inhibition of human beta-glucocerebrosidase by Lineweaver-Burk double reciprocal plot methodMore data for this Ligand-Target Pair
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Genomics Research Center
Curated by ChEMBL
Genomics Research Center
Curated by ChEMBL
Affinity DataKi: 1.10E+3nMAssay Description:Non-competitive inhibition of human beta-glucocerebrosidase by Lineweaver-Burk double reciprocal plot methodMore data for this Ligand-Target Pair
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Genomics Research Center
Curated by ChEMBL
Genomics Research Center
Curated by ChEMBL
Affinity DataKi: 1.30E+3nMAssay Description:Competitive inhibition of human beta-glucocerebrosidase by Lineweaver-Burk double reciprocal plot methodMore data for this Ligand-Target Pair
Affinity DataKi: 2.00E+3nMAssay Description:Non-competitive inhibition of recombinant human alpha GAL-A using varying levels of 4-methylumbelliferyl alpha-D-galactopyranoside substrate at pH 7 ...More data for this Ligand-Target Pair
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Genomics Research Center
Curated by ChEMBL
Genomics Research Center
Curated by ChEMBL
Affinity DataKi: 3.20E+3nMAssay Description:Competitive inhibition of human beta-glucocerebrosidase by Lineweaver-Burk double reciprocal plot methodMore data for this Ligand-Target Pair
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Genomics Research Center
Curated by ChEMBL
Genomics Research Center
Curated by ChEMBL
Affinity DataKi: 4.10E+3nMAssay Description:Competitive inhibition of human beta-glucocerebrosidase by Lineweaver-Burk double reciprocal plot methodMore data for this Ligand-Target Pair
Affinity DataKi: >5.00E+3nMAssay Description:Binding affinity of the compound was evaluated against Coagulation factor XMore data for this Ligand-Target Pair
Affinity DataKi: >5.00E+3nMAssay Description:Binding affinity of the compound was evaluated against Coagulation factor XMore data for this Ligand-Target Pair
Affinity DataKi: >5.00E+3nMAssay Description:Binding affinity of the compound was evaluated against Coagulation factor XMore data for this Ligand-Target Pair
Affinity DataKi: >5.00E+3nMAssay Description:Binding affinity of the compound was evaluated against Coagulation factor XMore data for this Ligand-Target Pair
Affinity DataKi: >5.00E+3nMAssay Description:Binding affinity of the compound was evaluated against Coagulation factor XMore data for this Ligand-Target Pair
Affinity DataKi: >5.00E+3nMAssay Description:Binding affinity of the compound was evaluated against Coagulation factor XMore data for this Ligand-Target Pair
Affinity DataKi: >5.00E+3nMAssay Description:Binding affinity of the compound was evaluated against Coagulation factor XMore data for this Ligand-Target Pair
Affinity DataKi: >5.00E+3nMAssay Description:Binding affinity of the compound was evaluated against Coagulation factor XMore data for this Ligand-Target Pair
Affinity DataKi: >5.00E+3nMAssay Description:Binding affinity of the compound was evaluated against Coagulation factor XMore data for this Ligand-Target Pair
Affinity DataKi: >5.00E+3nMAssay Description:Binding affinity of the compound was evaluated against Coagulation factor XMore data for this Ligand-Target Pair
Affinity DataKi: >5.00E+3nMAssay Description:Binding affinity of the compound was evaluated against Coagulation factor XMore data for this Ligand-Target Pair
Affinity DataKi: >5.00E+3nMAssay Description:Binding affinity of the compound was evaluated against Coagulation factor XMore data for this Ligand-Target Pair
Affinity DataKi: >5.00E+3nMAssay Description:Binding affinity of the compound was evaluated against Coagulation factor XMore data for this Ligand-Target Pair
Affinity DataKi: 7.70E+3nMAssay Description:Non-competitive inhibition of recombinant human alpha GAL-A using varying levels of 4-methylumbelliferyl alpha-D-galactopyranoside substrate at pH 4....More data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMAssay Description:This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluoresence...More data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMAssay Description:This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluoresence...More data for this Ligand-Target Pair
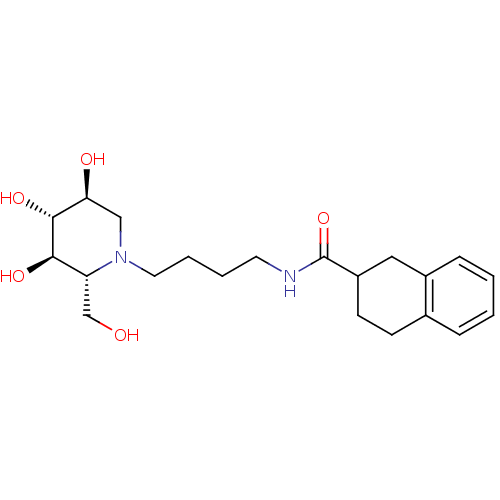
Affinity DataIC50: 0.370nMAssay Description:Concentration required to inhibit the VCAM-Very late antigen4 (VLA4) interaction in Ramos cell-based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluoresence...More data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluoresence...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluoresence...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Concentration required to inhibit the VCAM-Very late antigen4 (VLA4) interaction in Ramos cell-based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Concentration required to inhibit the VCAM-Very late antigen4 (VLA4) interaction in ELISA cell-based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Concentration required to inhibit the VCAM-Very late antigen4 (VLA4) interaction in ELISA cell-based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Concentration required to inhibit the VCAM-Very late antigen4 (VLA4) interaction in Ramos cell-based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.60nMAssay Description:This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluoresence...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Concentration required to inhibit the VCAM-Very late antigen4 (VLA4) interaction in Ramos cell-based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Concentration required to inhibit the VCAM-Very late antigen4 (VLA4) interaction in ELISA cell-based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Concentration required to inhibit the VCAM-Very late antigen4 (VLA4) interaction in ELISA cell-based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 6.5nMAssay Description:Concentration required to inhibit the VCAM-Very late antigen4 (VLA4) interaction in ELISA cell-based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:Inhibition of human DGAT1More data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluoresence...More data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:Concentration required to inhibit the VCAM-Very late antigen4 (VLA4) interaction in ELISA cell-based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluoresence...More data for this Ligand-Target Pair
Affinity DataIC50: 11nMAssay Description:Concentration required to inhibit the VCAM-Very late antigen4 (VLA4) interaction in ELISA cell-based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:Concentration required to inhibit the VCAM-Very late antigen4 (VLA4) interaction in Ramos cell-based assayMore data for this Ligand-Target Pair


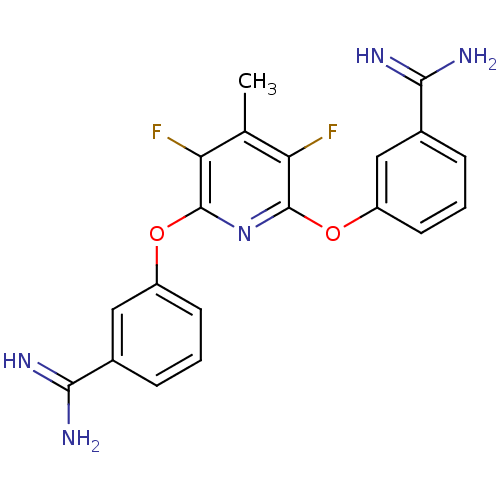

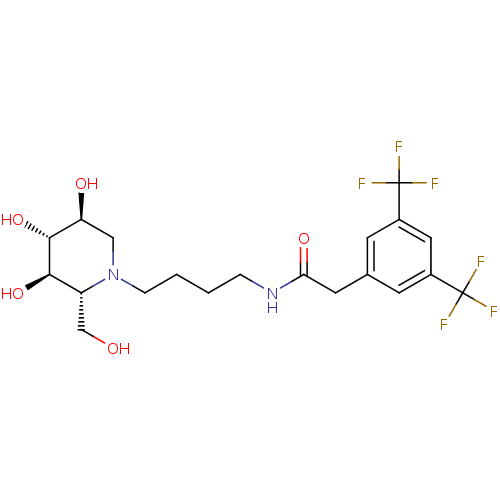


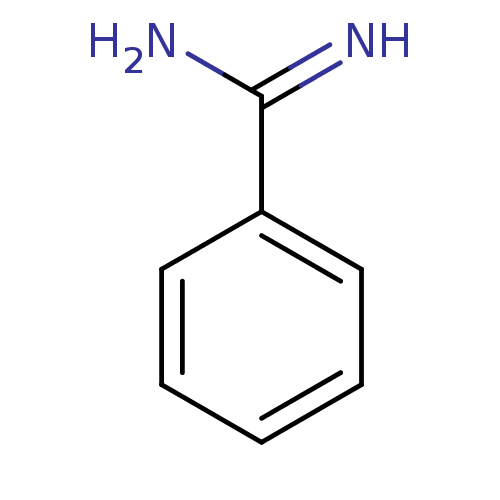
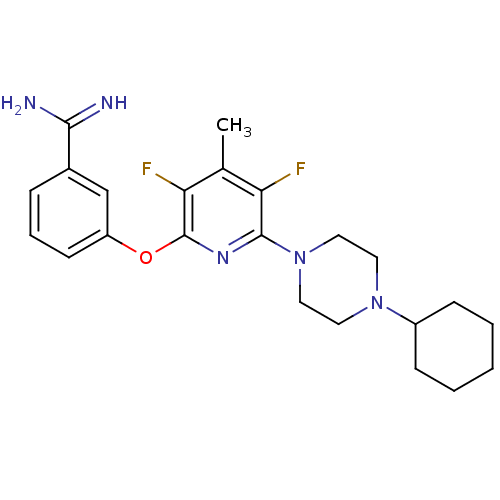

 3D Structure (crystal)
3D Structure (crystal)