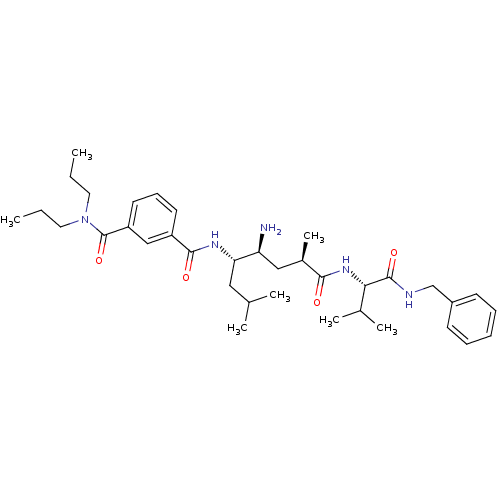
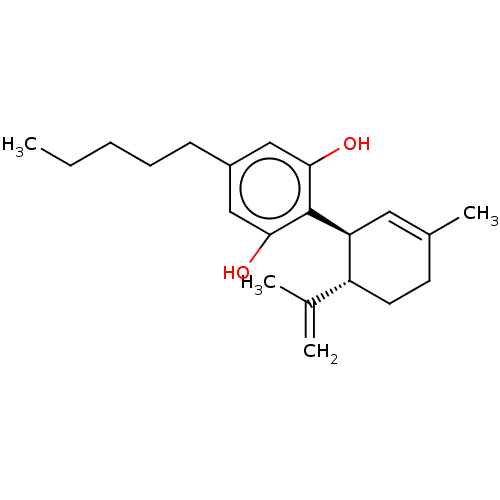
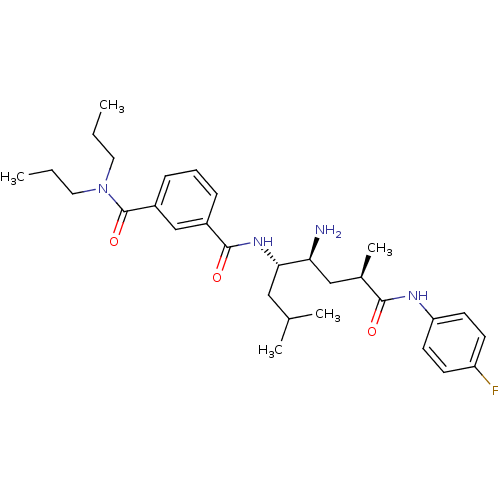
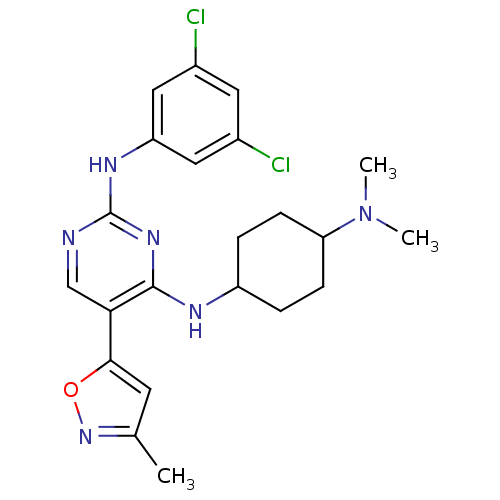
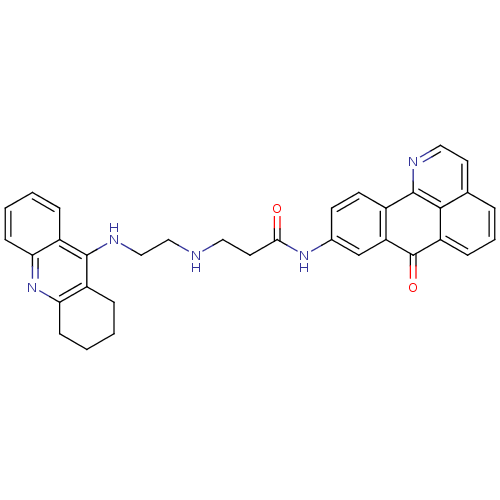
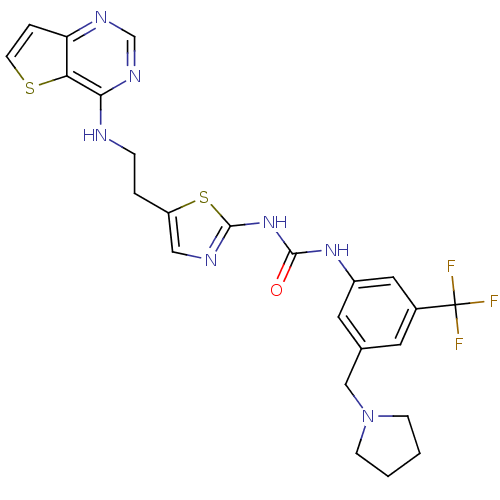
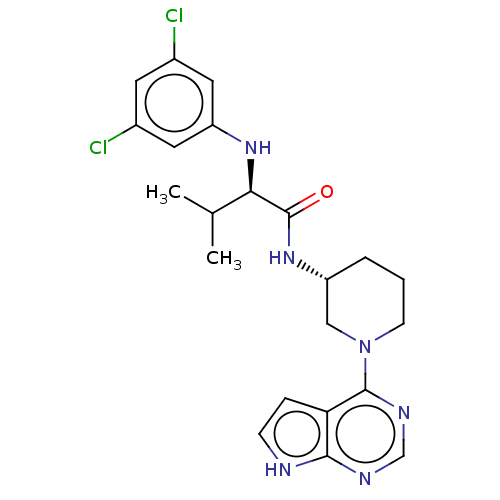
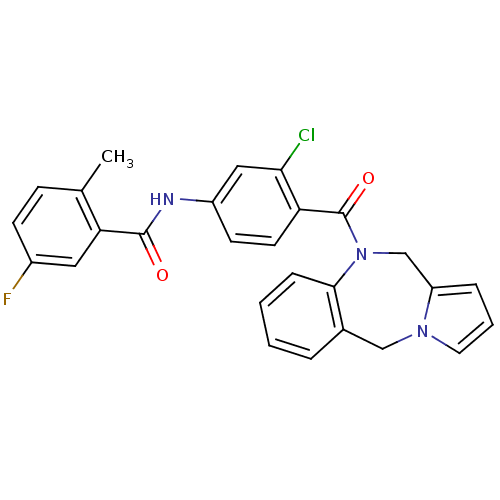
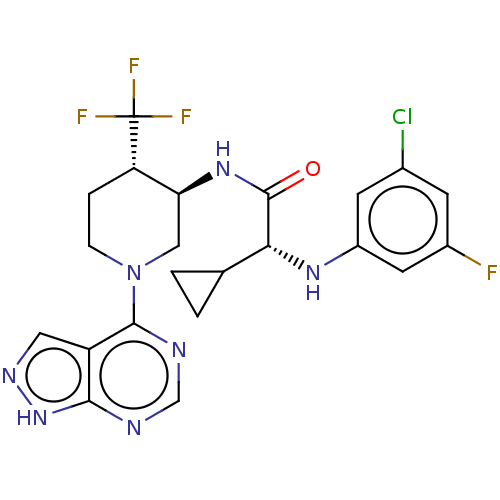
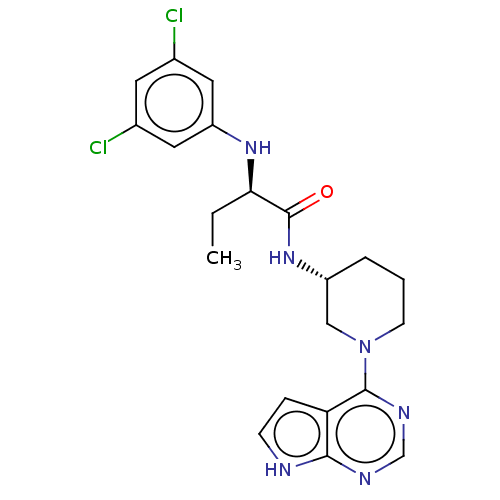
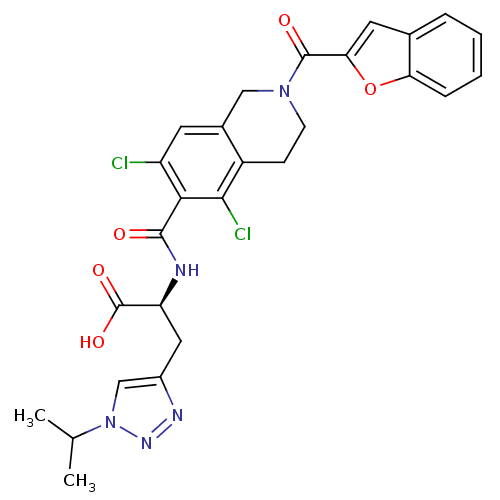
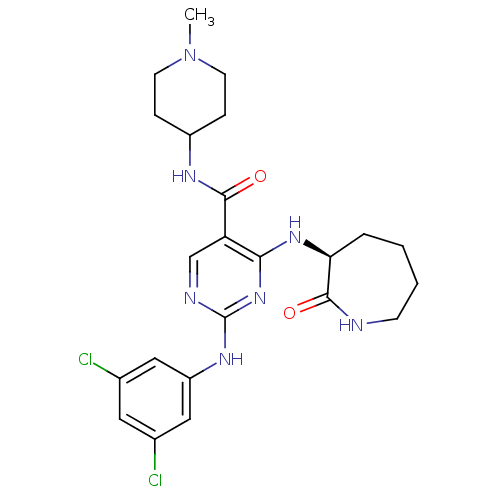
Affinity DataKi: 11nMAssay Description:Displacement of [3H]CP 55940 from human CB1 receptor after 1 hr by liquid scintillation spectrometryMore data for this Ligand-Target Pair
Affinity DataKi: 26nM ΔG°: -42.9kJ/molepH: 4.5 T: 2°CAssay Description:Compounds were tested for their ability to inhibit BACE-1 hydrolysis of the internally quenched fluorescent substrate FS-2. Reactions were initiated ...More data for this Ligand-Target Pair
Affinity DataKi: 33nM ΔG°: -42.3kJ/molepH: 4.5 T: 2°CAssay Description:Compounds were tested for their ability to inhibit BACE-1 hydrolysis of the internally quenched fluorescent substrate FS-2. Reactions were initiated ...More data for this Ligand-Target Pair
Affinity DataKi: 33nMAssay Description:Displacement of [3H]CP 55940 from human CB1 receptor after 1 hr by liquid scintillation spectrometryMore data for this Ligand-Target Pair
Affinity DataKi: 71nM ΔG°: -40.4kJ/molepH: 4.5 T: 2°CAssay Description:Compounds were tested for their ability to inhibit BACE-1 hydrolysis of the internally quenched fluorescent substrate FS-2. Reactions were initiated ...More data for this Ligand-Target Pair
Affinity DataKi: 120nM ΔG°: -39.1kJ/molepH: 4.5 T: 2°CAssay Description:Compounds were tested for their ability to inhibit BACE-1 hydrolysis of the internally quenched fluorescent substrate FS-2. Reactions were initiated ...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
School Of Chemistry & Chemical Engineering Of Guangxi Normal University
Curated by ChEMBL
School Of Chemistry & Chemical Engineering Of Guangxi Normal University
Curated by ChEMBL
Affinity DataKi: 260nMAssay Description:Non-competitive inhibition of Electrophorus electricus AChE assessed as hydrolysis of acetylthiocholineiodide after 15 mins incubation by spectrophot...More data for this Ligand-Target Pair
Affinity DataKi: 842nMAssay Description:Displacement of [3H]-HU-243 from CB1 receptor in Sabra rat brain synaptosomes after 90 minsMore data for this Ligand-Target Pair
Affinity DataKi: 1.30E+3nM ΔG°: -33.3kJ/molepH: 4.5 T: 2°CAssay Description:Compounds were tested for their ability to inhibit BACE-1 hydrolysis of the internally quenched fluorescent substrate FS-2. Reactions were initiated ...More data for this Ligand-Target Pair
Affinity DataKi: 3.00E+3nM ΔG°: -31.2kJ/molepH: 4.5 T: 2°CAssay Description:Compounds were tested for their ability to inhibit BACE-1 hydrolysis of the internally quenched fluorescent substrate FS-2. Reactions were initiated ...More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [3H]-HU-243 from CB1 receptor in Sabra rat brain synaptosomes after 90 minsMore data for this Ligand-Target Pair
Affinity DataKi: 1.13E+4nM ΔG°: -28.0kJ/molepH: 4.5 T: 2°CAssay Description:Compounds were tested for their ability to inhibit BACE-1 hydrolysis of the internally quenched fluorescent substrate FS-2. Reactions were initiated ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMAssay Description:Inhibition of Sky (unknown origin) by ELISA kinase assay in presence of 60 uM ATPMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of BTK (unknown origin) incubated 30 mins by FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Reversible inhibition of recombinant full-length N-terminal 6-His-tagged human BTK expressed in baculovirus infected Sf21 insect cells using fluoresc...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:Inhibition of LFA1/ICAM1 interaction in human Hut-78 cellsMore data for this Ligand-Target Pair
TargetIntegrin alpha-L/Integrin beta-2/Intercellular adhesion molecule 1(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 1.20nMAssay Description:Antagonist activity at LFA-1/ICAM-1 in human HuT-78 T-cells assessed as inhibition of cell adhesion after 1 hr by p-nitrophenyl n-acetyl-beta-D-gluco...More data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:Inhibition of LFA1 by ELISAMore data for this Ligand-Target Pair
TargetVasopressin V1a receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Displacement of [3H]-Arg-vasopressin from human recombinant vasopressin V1a receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein kinase FLT3(Homo sapiens (Human))
Sichuan University/Collaborative Innovation Center Of Biotherapy
Curated by ChEMBL
Sichuan University/Collaborative Innovation Center Of Biotherapy
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of recombinant human N-terminal GST-tagged FLT3 (564 to end residues) expressed by baculovirus in Sf21 insect cells measured after 10 mins...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of Sky (unknown origin) by ELISA kinase assay in presence of 60 uM ATPMore data for this Ligand-Target Pair
TargetStimulator of interferon genes protein [138-378](Mus musculus (Mouse))
Janssen Biotech
US Patent
Janssen Biotech
US Patent
Affinity DataIC50: <2nMAssay Description:The human STING SPA binding assay measures displacement of tritium labeled 2′,3′cGAMP (cyclic (guanosine-(2′→5′)-monoph...More data for this Ligand-Target Pair
TargetIntegrin alpha-L/Integrin beta-2/Intercellular adhesion molecule 1(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Antagonist activity at LFA-1/ICAM-1 in human HuT-78 T-cells assessed as inhibition of cell adhesion after 1 hr by p-nitrophenyl n-acetyl-beta-D-gluco...More data for this Ligand-Target Pair
Affinity DataIC50: 2.98nMAssay Description:Inhibition of human recombinant ICAM-1 adhesion into human Jurkat cells after 1 hrMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of Sky (unknown origin) by ELISA kinase assay in presence of 60 uM ATPMore data for this Ligand-Target Pair
TargetIntegrin alpha-L/Integrin beta-2/Intercellular adhesion molecule 1(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Antagonist activity at LFA-1/ICAM-1 in human HuT-78 T-cells assessed as inhibition of cell adhesion after 1 hr by p-nitrophenyl n-acetyl-beta-D-gluco...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
School Of Chemistry & Chemical Engineering Of Guangxi Normal University
Curated by ChEMBL
School Of Chemistry & Chemical Engineering Of Guangxi Normal University
Curated by ChEMBL
Affinity DataIC50: 3.40nMAssay Description:Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's methodMore data for this Ligand-Target Pair
TargetIntegrin alpha-L/Integrin beta-2/Intercellular adhesion molecule 1(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Antagonist activity at LFA-1/ICAM-1 in human HuT-78 T-cells assessed as inhibition of cell adhesion after 1 hr by p-nitrophenyl n-acetyl-beta-D-gluco...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibition of BTK (unknown origin) incubated 30 mins by FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibition of BTK (unknown origin) incubated 30 mins by FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 4nMpH: 7.2 T: 2°CAssay Description:Compounds were diluted into assay buffer containing Aurora kinase and FAM-PKAtide. The kinase reaction was initiated by adding ATP in assay buffer. T...More data for this Ligand-Target Pair
TargetVasopressin V1a receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Displacement of [3H]-Arg-vasopressin from human recombinant vasopressin V1a receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of Aurora B after 60 minsMore data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein kinase FLT3(Homo sapiens (Human))
Sichuan University/Collaborative Innovation Center Of Biotherapy
Curated by ChEMBL
Sichuan University/Collaborative Innovation Center Of Biotherapy
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of recombinant human N-terminal GST-tagged FLT3 (564 to end residues) expressed by baculovirus in Sf21 insect cells measured after 10 mins...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of BTK (unknown origin) incubated 30 mins by FRET assayMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Displacement of [3H]-Arg-vasopressin from human recombinant vasopressin V2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of BTK (unknown origin) incubated 30 mins by FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of BTK (unknown origin) incubated 30 mins by FRET assayMore data for this Ligand-Target Pair
TargetIntegrin beta-2/Intercellular adhesion molecule 1(Homo sapiens (Human))
Sunesis Pharmaceuticals
Curated by ChEMBL
Sunesis Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of LFA1/ICAM1 interaction in human Hut-78 cells by cell migration assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of LFA1/ICAM1 interaction in human Hut-78 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of LFA1/ICAM1 interaction in human Hut-78 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of Sky (unknown origin) by ELISA kinase assay in presence of 60 uM ATPMore data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein kinase FLT3(Homo sapiens (Human))
Sichuan University/Collaborative Innovation Center Of Biotherapy
Curated by ChEMBL
Sichuan University/Collaborative Innovation Center Of Biotherapy
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Inhibition of recombinant human N-terminal GST-tagged FLT3 (564 to end residues) expressed by baculovirus in Sf21 insect cells measured after 10 mins...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of Aurora B after 60 minsMore data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein kinase FLT3(Homo sapiens (Human))
Sichuan University/Collaborative Innovation Center Of Biotherapy
Curated by ChEMBL
Sichuan University/Collaborative Innovation Center Of Biotherapy
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Inhibition of recombinant human N-terminal GST-tagged FLT3 (564 to end residues) expressed by baculovirus in Sf21 insect cells measured after 10 mins...More data for this Ligand-Target Pair
TargetIntegrin alpha-L/Integrin beta-2/Intercellular adhesion molecule 1(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Antagonist activity at LFA-1/ICAM-1 in human HuT-78 T-cells assessed as inhibition of cell adhesion after 1 hr by p-nitrophenyl n-acetyl-beta-D-gluco...More data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:Inhibition of LFA1/ICAM1 interaction in human Hut-78 cellsMore data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein kinase FLT3(Homo sapiens (Human))
Sichuan University/Collaborative Innovation Center Of Biotherapy
Curated by ChEMBL
Sichuan University/Collaborative Innovation Center Of Biotherapy
Curated by ChEMBL
Affinity DataIC50: 8nMAssay Description:Inhibition of recombinant human N-terminal GST-tagged FLT3 (564 to end residues) expressed by baculovirus in Sf21 insect cells measured after 10 mins...More data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein kinase FLT3(Homo sapiens (Human))
Sichuan University/Collaborative Innovation Center Of Biotherapy
Curated by ChEMBL
Sichuan University/Collaborative Innovation Center Of Biotherapy
Curated by ChEMBL
Affinity DataIC50: 8nMAssay Description:Inhibition of recombinant human N-terminal GST-tagged FLT3 (564 to end residues) expressed by baculovirus in Sf21 insect cells measured after 10 mins...More data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Inhibition of Aurora B after 60 minsMore data for this Ligand-Target Pair

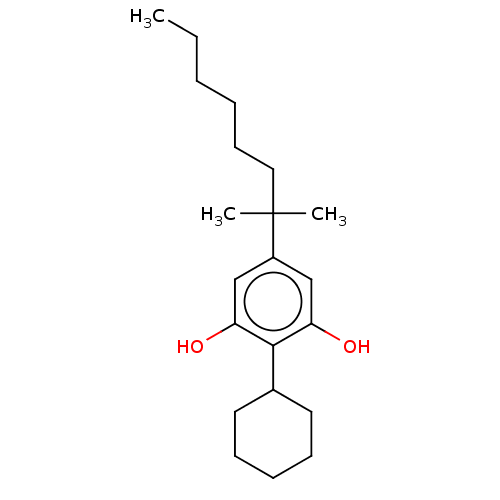
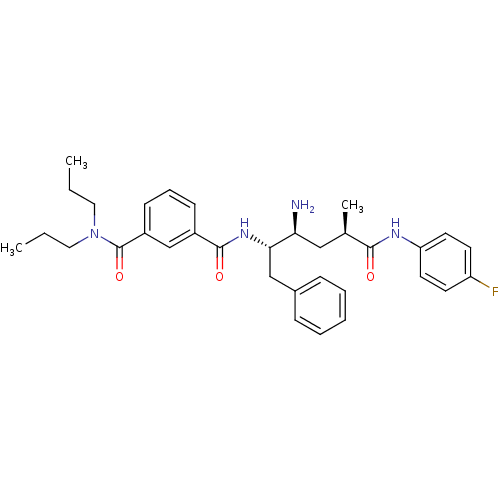
 3D Structure (crystal)
3D Structure (crystal)