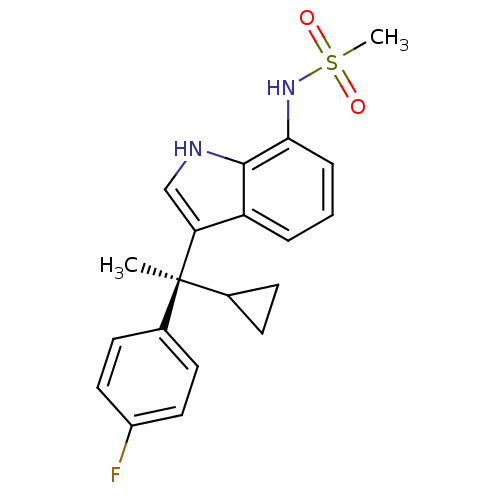
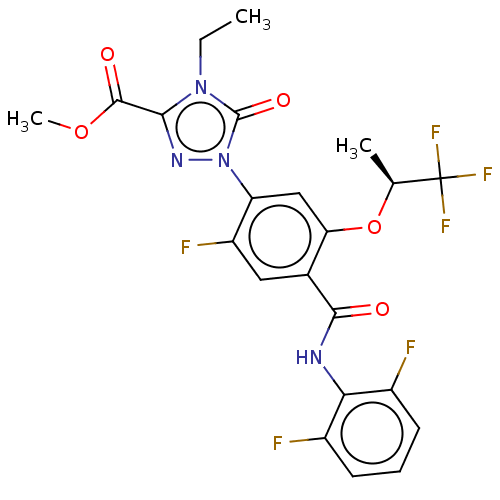
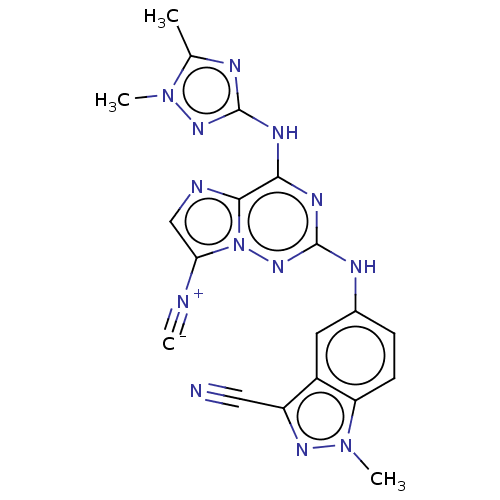
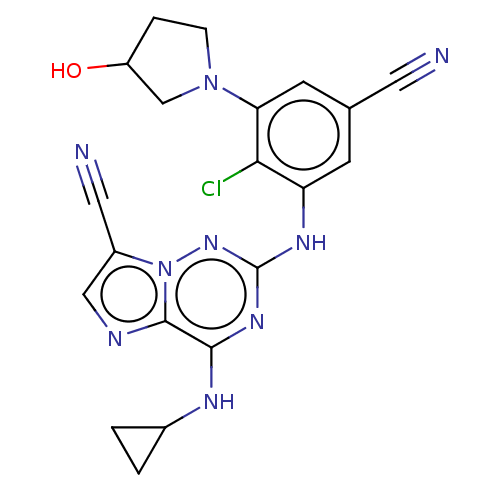
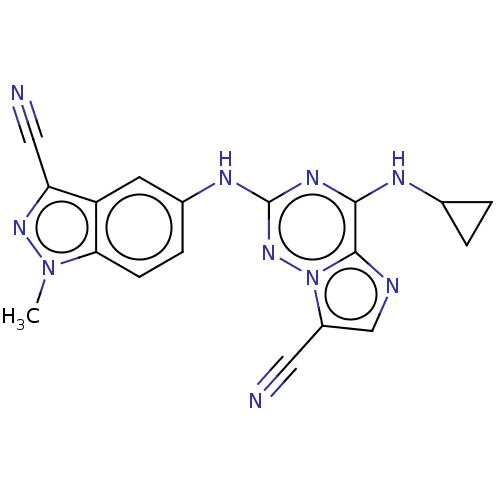
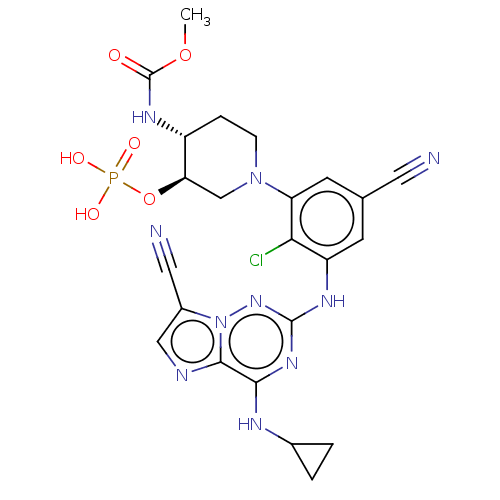
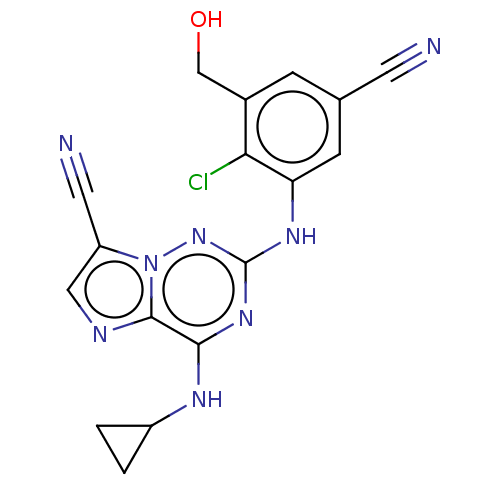
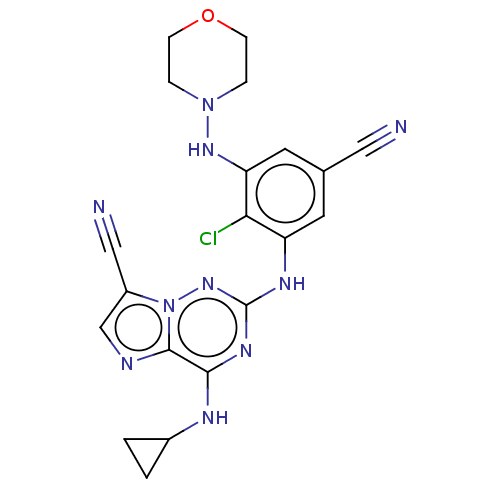
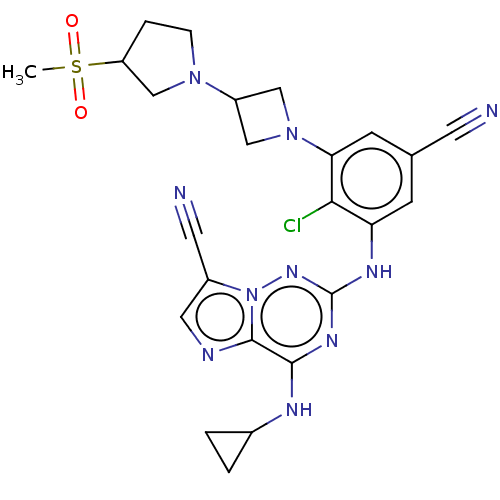
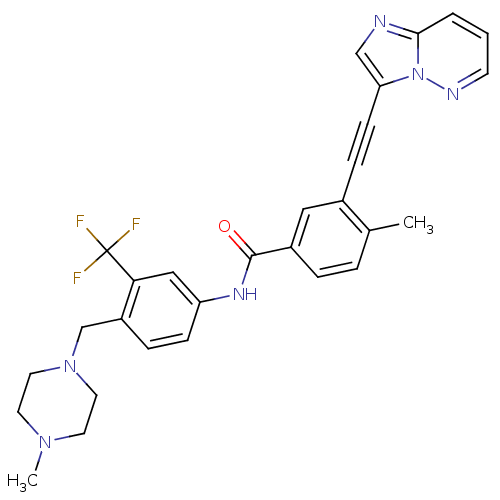
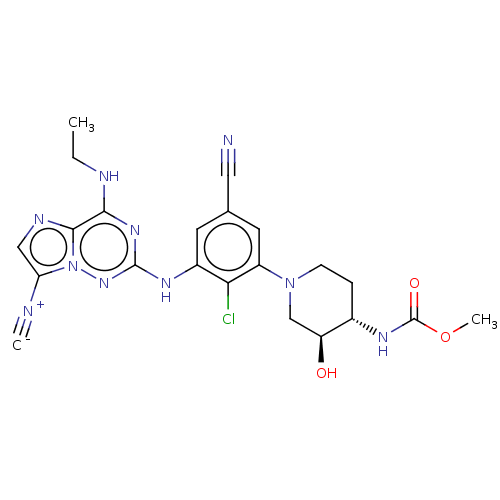
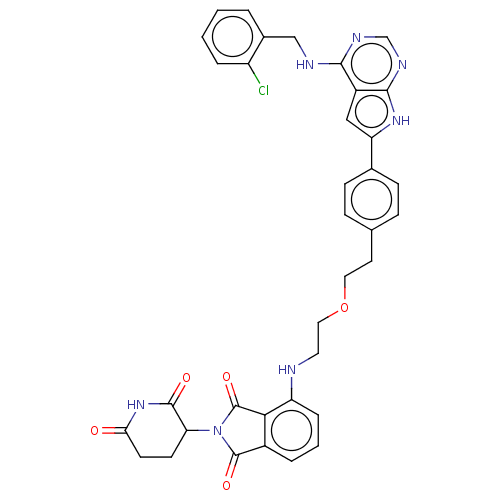
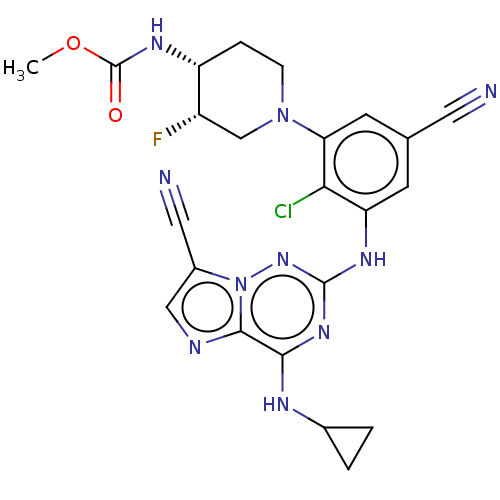
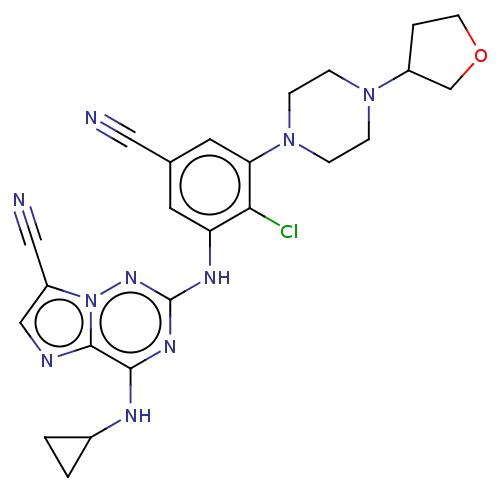
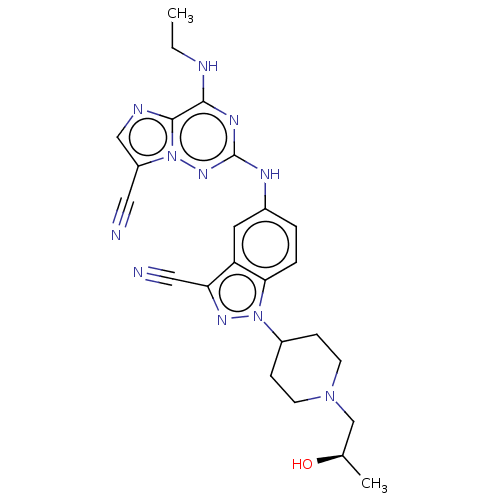
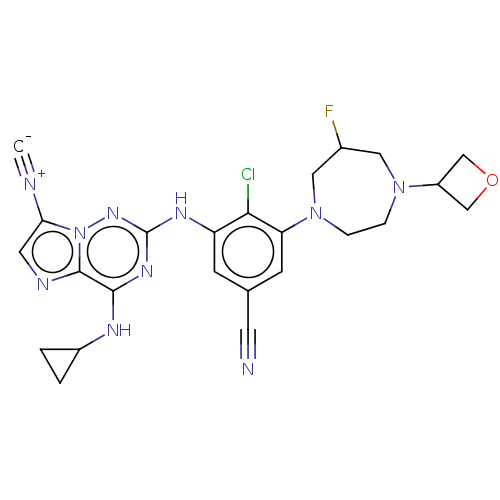
Affinity DataKi: 0.494nMAssay Description:Binding affinity to human mineralocorticoid receptorMore data for this Ligand-Target Pair
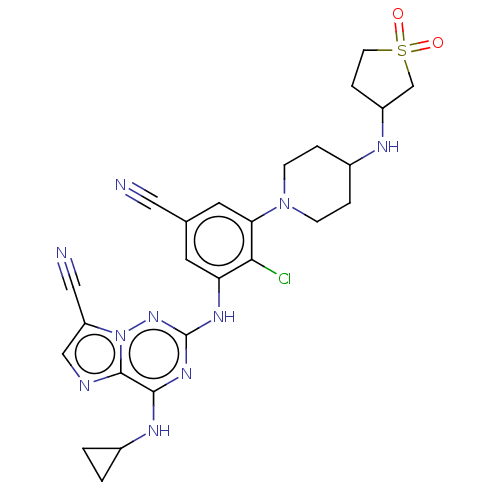
Affinity DataKi: 1.56nMAssay Description:Binding affinity to human mineralocorticoid receptorMore data for this Ligand-Target Pair
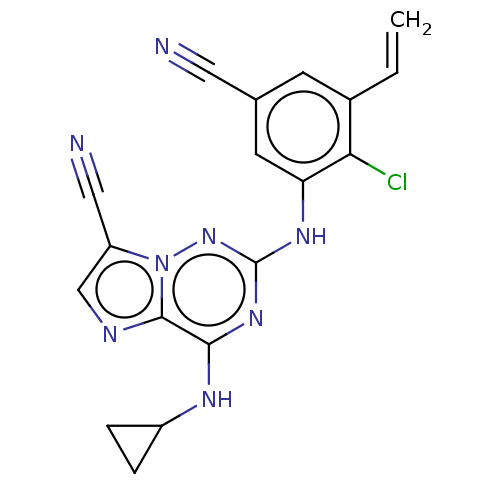
Affinity DataKi: 2.25nMAssay Description:Binding affinity to human mineralocorticoid receptorMore data for this Ligand-Target Pair
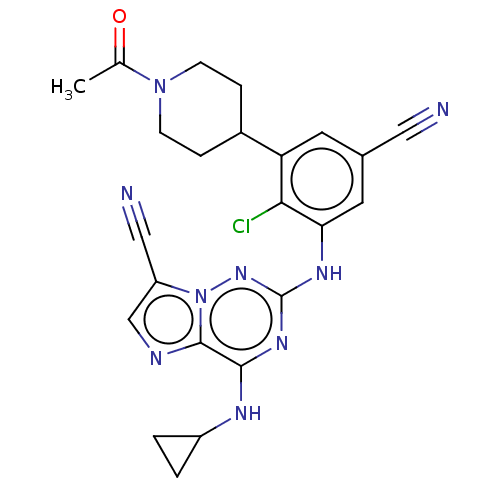
Affinity DataKi: 2.32nMAssay Description:Binding affinity to human mineralocorticoid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 7.5nMAssay Description:Binding affinity to human mineralocorticoid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 8.90nMAssay Description:Binding affinity to human glucocorticoid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 32.6nMAssay Description:Binding affinity to human glucocorticoid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 39.4nMAssay Description:Binding affinity to human androgen receptorMore data for this Ligand-Target Pair
Affinity DataKi: 124nMAssay Description:Binding affinity to human mineralocorticoid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 137nMAssay Description:Binding affinity to human progesterone receptorMore data for this Ligand-Target Pair
Affinity DataKi: 163nMAssay Description:Binding affinity to human progesterone receptorMore data for this Ligand-Target Pair
Affinity DataKi: 167nMAssay Description:Binding affinity to human glucocorticoid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 313nMAssay Description:Binding affinity to human progesterone receptorMore data for this Ligand-Target Pair
Affinity DataKi: 387nMAssay Description:Binding affinity to human glucocorticoid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 400nMAssay Description:Binding affinity to human progesterone receptorMore data for this Ligand-Target Pair
Affinity DataKi: 590nMAssay Description:Binding affinity to human androgen receptorMore data for this Ligand-Target Pair
Affinity DataKi: 700nMAssay Description:Binding affinity to human androgen receptorMore data for this Ligand-Target Pair
Affinity DataKi: 849nMAssay Description:Binding affinity to human androgen receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.10E+3nMAssay Description:Binding affinity to human estrogen receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.10E+3nMAssay Description:Binding affinity to human estrogen receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.10E+3nMAssay Description:Binding affinity to human estrogen receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.10E+3nMAssay Description:Binding affinity to human estrogen receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.10E+3nMAssay Description:Binding affinity to human estrogen receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1.76E+3nMAssay Description:Binding affinity to human glucocorticoid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 8.89E+3nMAssay Description:Binding affinity to human androgen receptorMore data for this Ligand-Target Pair
Affinity DataKi: 2.94E+4nMAssay Description:Binding affinity to human progesterone receptorMore data for this Ligand-Target Pair
TargetDihydroorotate dehydrogenase (quinone), mitochondrial(Homo sapiens (Human))
Bayer Aktiengellschaft
US Patent
Bayer Aktiengellschaft
US Patent
Affinity DataIC50: 0nMAssay Description:The enzymatic assay couples DHODH activity with bleaching of the dye 2,6-dichlorophenolindophenol (DCIP) (Knecht and Loffler, 1998; Miller et al., 19...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0300nMpH: 7.4 T: 2°CAssay Description:The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0700nMpH: 7.4 T: 2°CAssay Description:The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0900nMpH: 7.4 T: 2°CAssay Description:The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0900nMpH: 7.4 T: 2°CAssay Description:The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th...More data for this Ligand-Target Pair
Affinity DataIC50: 0.110nMpH: 7.4 T: 2°CAssay Description:The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th...More data for this Ligand-Target Pair
Affinity DataIC50: 0.110nMpH: 7.4 T: 2°CAssay Description:The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th...More data for this Ligand-Target Pair
Affinity DataIC50: 0.110nMpH: 7.4 T: 2°CAssay Description:The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th...More data for this Ligand-Target Pair
Affinity DataIC50: 0.110nMAssay Description:Binding affinity to human HCK using KVEKIGEGTYGVVYK as substrate by radiometric hotspot kinase assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.130nMpH: 7.4 T: 2°CAssay Description:The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th...More data for this Ligand-Target Pair
Affinity DataIC50: 0.130nMpH: 7.4 T: 2°CAssay Description:The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th...More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor [1-18,20-745,747-749,751-1210](Homo sapiens (Human))
Arvinas Operations
US Patent
Arvinas Operations
US Patent
Affinity DataIC50: 0.140nMAssay Description:All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A...More data for this Ligand-Target Pair
Affinity DataIC50: 0.140nMpH: 7.4 T: 2°CAssay Description:The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th...More data for this Ligand-Target Pair
Affinity DataIC50: 0.150nMpH: 7.4 T: 2°CAssay Description:The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th...More data for this Ligand-Target Pair
Affinity DataIC50: 0.150nMpH: 7.4 T: 2°CAssay Description:The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th...More data for this Ligand-Target Pair
Affinity DataIC50: 0.160nMpH: 7.4 T: 2°CAssay Description:The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th...More data for this Ligand-Target Pair
Affinity DataIC50: 0.160nMpH: 7.4 T: 2°CAssay Description:The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th...More data for this Ligand-Target Pair
Affinity DataIC50: 0.160nMpH: 7.4 T: 2°CAssay Description:The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th...More data for this Ligand-Target Pair
Affinity DataIC50: 0.160nMAssay Description:Binding affinity to LYN (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 0.170nMpH: 7.4 T: 2°CAssay Description:The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th...More data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMpH: 7.4 T: 2°CAssay Description:The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th...More data for this Ligand-Target Pair
Affinity DataIC50: 0.210nMpH: 7.4 T: 2°CAssay Description:The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th...More data for this Ligand-Target Pair
Affinity DataIC50: 0.210nMpH: 7.4 T: 2°CAssay Description:The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th...More data for this Ligand-Target Pair

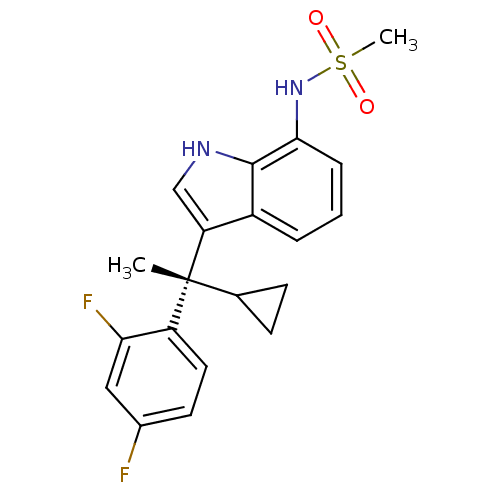
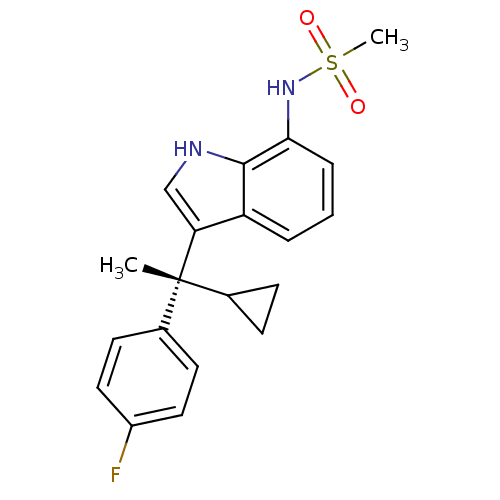

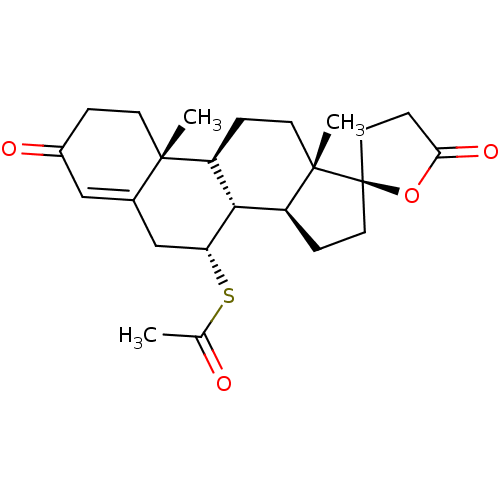
 3D Structure (crystal)
3D Structure (crystal)