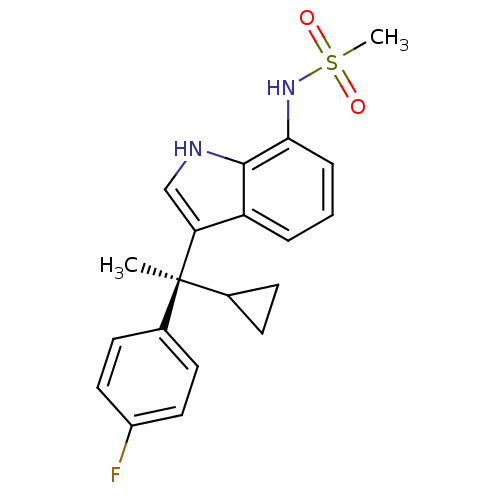
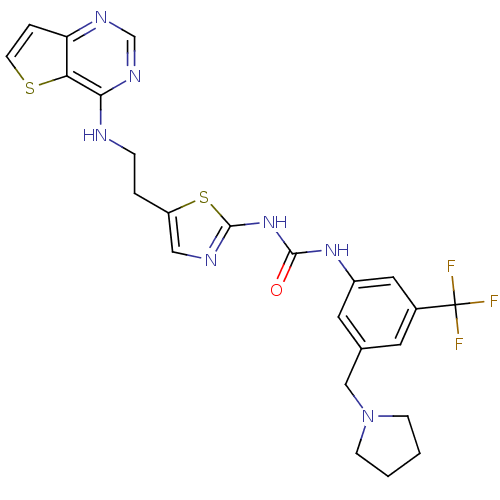
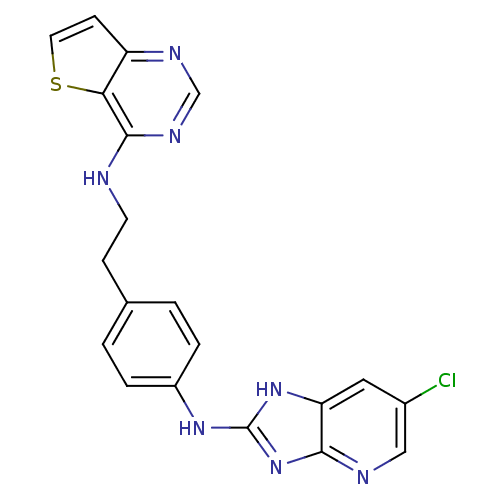
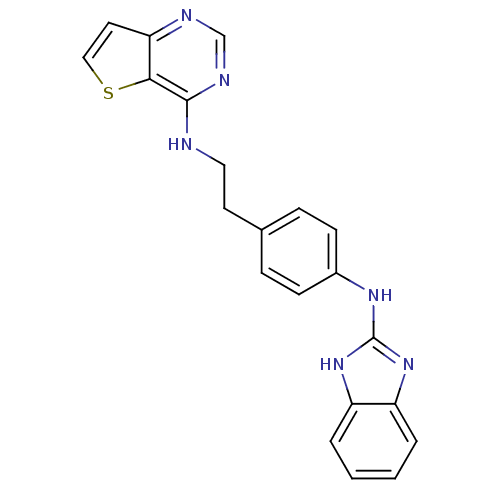
Affinity DataKi: 0.494nMAssay Description:Binding affinity to human mineralocorticoid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1.56nMAssay Description:Binding affinity to human mineralocorticoid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 2.25nMAssay Description:Binding affinity to human mineralocorticoid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 2.32nMAssay Description:Binding affinity to human mineralocorticoid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 7.5nMAssay Description:Binding affinity to human mineralocorticoid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 8.90nMAssay Description:Binding affinity to human glucocorticoid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 32.6nMAssay Description:Binding affinity to human glucocorticoid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 39.4nMAssay Description:Binding affinity to human androgen receptorMore data for this Ligand-Target Pair
Affinity DataKi: 124nMAssay Description:Binding affinity to human mineralocorticoid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 137nMAssay Description:Binding affinity to human progesterone receptorMore data for this Ligand-Target Pair
Affinity DataKi: 163nMAssay Description:Binding affinity to human progesterone receptorMore data for this Ligand-Target Pair
Affinity DataKi: 167nMAssay Description:Binding affinity to human glucocorticoid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 313nMAssay Description:Binding affinity to human progesterone receptorMore data for this Ligand-Target Pair
Affinity DataKi: 387nMAssay Description:Binding affinity to human glucocorticoid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 400nMAssay Description:Binding affinity to human progesterone receptorMore data for this Ligand-Target Pair
Affinity DataKi: 590nMAssay Description:Binding affinity to human androgen receptorMore data for this Ligand-Target Pair
Affinity DataKi: 700nMAssay Description:Binding affinity to human androgen receptorMore data for this Ligand-Target Pair
Affinity DataKi: 849nMAssay Description:Binding affinity to human androgen receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.10E+3nMAssay Description:Binding affinity to human estrogen receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.10E+3nMAssay Description:Binding affinity to human estrogen receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.10E+3nMAssay Description:Binding affinity to human estrogen receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.10E+3nMAssay Description:Binding affinity to human estrogen receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.10E+3nMAssay Description:Binding affinity to human estrogen receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1.76E+3nMAssay Description:Binding affinity to human glucocorticoid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 8.89E+3nMAssay Description:Binding affinity to human androgen receptorMore data for this Ligand-Target Pair
Affinity DataKi: 2.94E+4nMAssay Description:Binding affinity to human progesterone receptorMore data for this Ligand-Target Pair
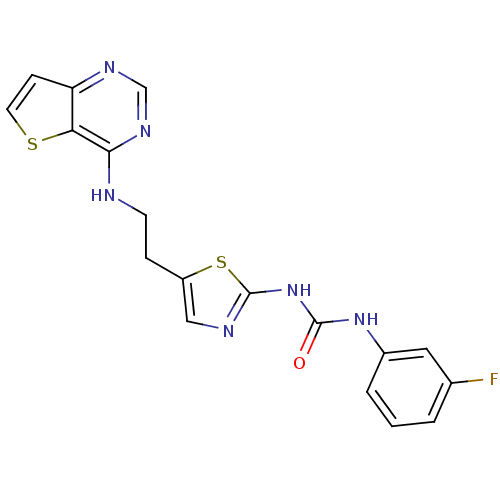
Affinity DataIC50: 4nMpH: 7.2 T: 2°CAssay Description:Compounds were diluted into assay buffer containing Aurora kinase and FAM-PKAtide. The kinase reaction was initiated by adding ATP in assay buffer. T...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of Aurora B after 60 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of Aurora B after 60 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Inhibition of Aurora B after 60 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 9nMpH: 7.2 T: 2°CAssay Description:Compounds were diluted into assay buffer containing Aurora kinase and FAM-PKAtide. The kinase reaction was initiated by adding ATP in assay buffer. T...More data for this Ligand-Target Pair
Affinity DataIC50: 9nMpH: 7.2 T: 2°CAssay Description:Compounds were diluted into assay buffer containing Aurora kinase and FAM-PKAtide. The kinase reaction was initiated by adding ATP in assay buffer. T...More data for this Ligand-Target Pair
Affinity DataIC50: 9nMAssay Description:Inhibition of Aurora A after 60 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of Aurora B after 60 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of Aurora B after 60 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 11nMpH: 7.2 T: 2°CAssay Description:Compounds were diluted into assay buffer containing Aurora kinase and FAM-PKAtide. The kinase reaction was initiated by adding ATP in assay buffer. T...More data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Compounds were diluted into assay buffer containing Aurora kinase and FAM-PKAtide. The kinase reaction was initiated by adding ATP in assay buffer. T...More data for this Ligand-Target Pair
Affinity DataIC50: 14nMAssay Description:Inhibition of Aurora B after 60 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 14nMAssay Description:Compounds were diluted into assay buffer containing Aurora kinase and FAM-PKAtide. The kinase reaction was initiated by adding ATP in assay buffer. T...More data for this Ligand-Target Pair
Affinity DataIC50: 17nMAssay Description:Inhibition of Aurora A after 60 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 18nMAssay Description:Compounds were diluted into assay buffer containing Aurora kinase and FAM-PKAtide. The kinase reaction was initiated by adding ATP in assay buffer. T...More data for this Ligand-Target Pair
Affinity DataIC50: 19nMAssay Description:Compounds were diluted into assay buffer containing Aurora kinase and FAM-PKAtide. The kinase reaction was initiated by adding ATP in assay buffer. T...More data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:Inhibition of Aurora A after 60 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 21nMAssay Description:Inhibition of Aurora B after 60 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 22nMpH: 7.2 T: 2°CAssay Description:Compounds were diluted into assay buffer containing Aurora kinase and FAM-PKAtide. The kinase reaction was initiated by adding ATP in assay buffer. T...More data for this Ligand-Target Pair
Affinity DataIC50: 22nMAssay Description:Compounds were diluted into assay buffer containing Aurora kinase and FAM-PKAtide. The kinase reaction was initiated by adding ATP in assay buffer. T...More data for this Ligand-Target Pair
Affinity DataIC50: 22nMAssay Description:Inhibition of Aurora A after 60 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 23nMpH: 7.2 T: 2°CAssay Description:Compounds were diluted into assay buffer containing Aurora kinase and FAM-PKAtide. The kinase reaction was initiated by adding ATP in assay buffer. T...More data for this Ligand-Target Pair
Affinity DataIC50: 28nMAssay Description:Inhibition of Aurora A after 60 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 28nMAssay Description:Inhibition of Aurora A after 60 minsMore data for this Ligand-Target Pair

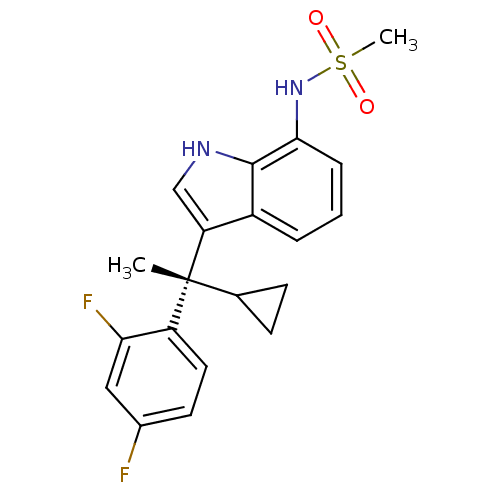
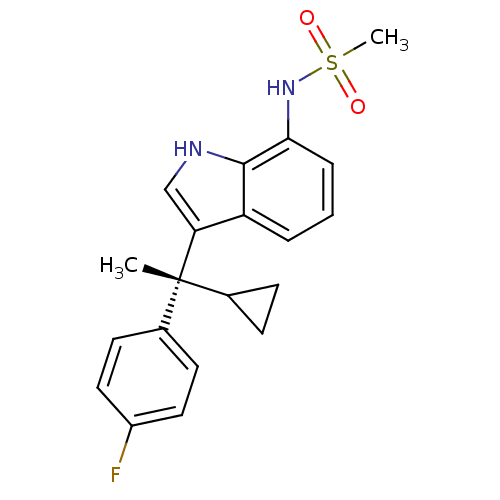

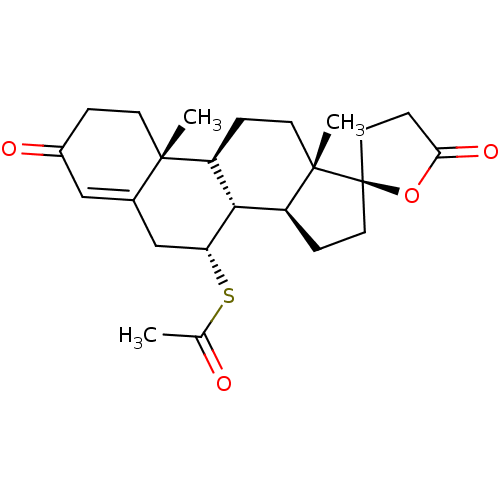
 3D Structure (crystal)
3D Structure (crystal)