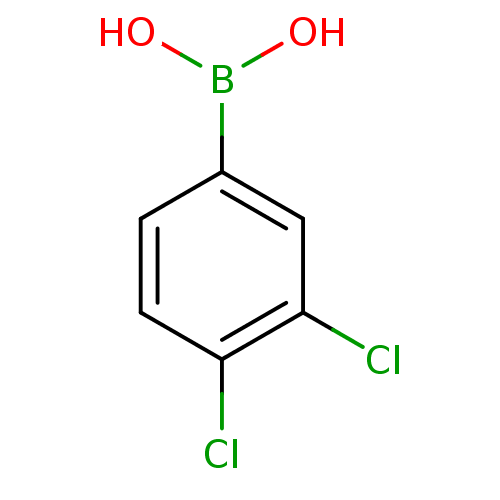
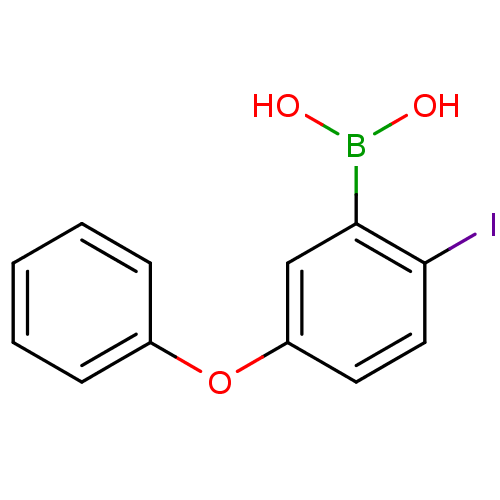
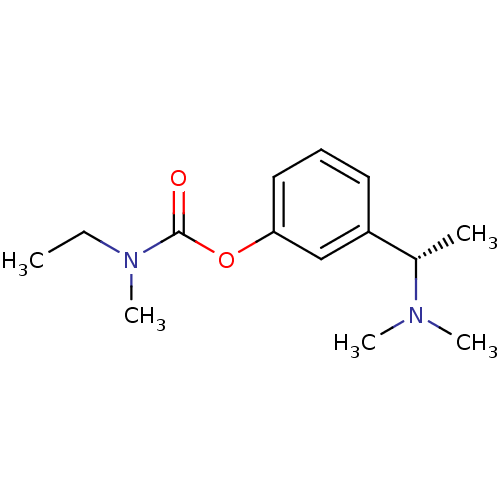
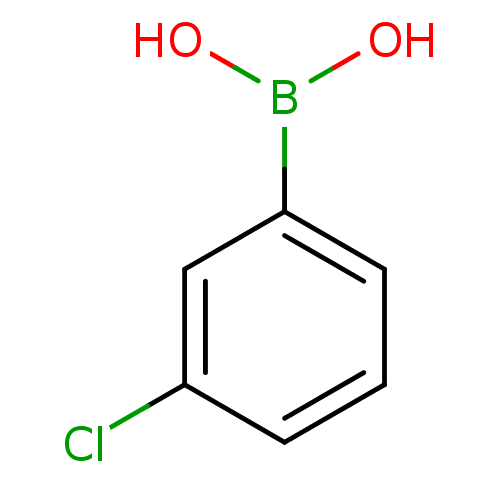
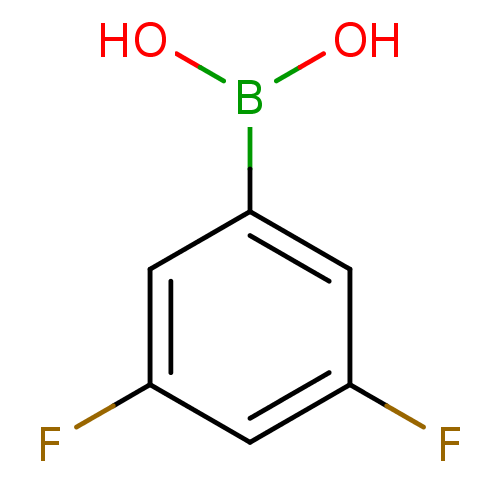
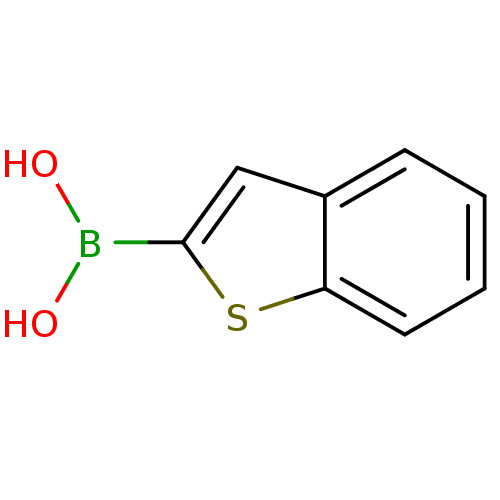
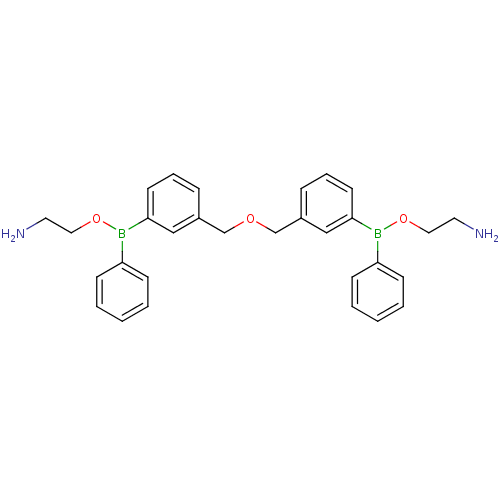
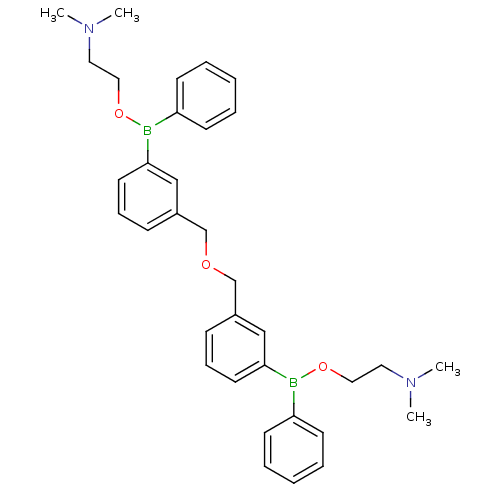
Affinity DataKi: 40nMAssay Description:Binding affinity to human recombinant carbonyl reductase 1 expressed in Escherichia coli assessed as NADPH oxidation using isatin as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 60nMAssay Description:Binding affinity to human recombinant carbonyl reductase 1 expressed in Escherichia coli assessed as NADPH oxidation using isatin as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 110nMAssay Description:Binding affinity to human recombinant carbonyl reductase 1 expressed in Escherichia coli assessed as NADPH oxidation using isatin as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 520nMAssay Description:Binding affinity to human recombinant carbonyl reductase 1 expressed in Escherichia coli assessed as NADPH oxidation using isatin as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 600nMAssay Description:Binding affinity to human recombinant carbonyl reductase 1 expressed in Escherichia coli assessed as NADPH oxidation using isatin as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 2.03E+3nMAssay Description:Binding affinity to human recombinant carbonyl reductase 1 expressed in Escherichia coli assessed as NADPH oxidation using isatin as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 3.43E+3nMAssay Description:Binding affinity to human recombinant carbonyl reductase 1 expressed in Escherichia coli assessed as NADPH oxidation using isatin as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 3.70nMAssay Description:Inhibition of human BChE using butyrylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition and measured every 30 se...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibition of human BChE using butyrylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition and measured every 30 se...More data for this Ligand-Target Pair
Affinity DataIC50: 6.30nMAssay Description:Inhibition of human BChE using butyrylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition and measured every 30 se...More data for this Ligand-Target Pair
Affinity DataIC50: 6.40nMAssay Description:Pseduo-irreversible inhibition of human BChE using butyrylthiocholineiodide as substrate preincubated for 30 mins followed by substrate addition and ...More data for this Ligand-Target Pair
Affinity DataIC50: 6.90nMAssay Description:Inhibition of human BChE using butyrylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition and measured every 30 se...More data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Inhibition of human BChE using butyrylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition and measured every 30 se...More data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Inhibition of human BChE using butyrylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition and measured every 30 se...More data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:Inhibition of human BChE using butyrylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition and measured every 30 se...More data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:Inhibition of human BChE using butyrylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition and measured every 30 se...More data for this Ligand-Target Pair
Affinity DataIC50: 31nMAssay Description:Inhibition of human BChE using butyrylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition and measured every 30 se...More data for this Ligand-Target Pair
Affinity DataIC50: 32nMAssay Description:Inhibition of human BChE using butyrylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition and measured every 30 se...More data for this Ligand-Target Pair
Affinity DataIC50: 47nMAssay Description:Inhibition of human BChE using butyrylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition and measured every 30 se...More data for this Ligand-Target Pair
Affinity DataIC50: 48nMAssay Description:Inhibition of human BChE using butyrylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition and measured every 30 se...More data for this Ligand-Target Pair
Affinity DataIC50: 50nMAssay Description:Inhibition of human BChE using butyrylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition and measured every 30 se...More data for this Ligand-Target Pair
Affinity DataIC50: 50nMAssay Description:Inhibition of human BChE using butyrylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition and measured every 30 se...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Julius Maximilian University Of W�Rzburg
Curated by ChEMBL
Julius Maximilian University Of W�Rzburg
Curated by ChEMBL
Affinity DataIC50: 58nMAssay Description:Inhibition of human AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and measured every 30 se...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Julius Maximilian University Of W�Rzburg
Curated by ChEMBL
Julius Maximilian University Of W�Rzburg
Curated by ChEMBL
Affinity DataIC50: 63nMAssay Description:Inhibition of human AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and measured every 30 se...More data for this Ligand-Target Pair
Affinity DataIC50: 77nMAssay Description:Inhibition of human BChE using butyrylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition and measured every 30 se...More data for this Ligand-Target Pair
Affinity DataIC50: 79nMAssay Description:Inhibition of human BChE using butyrylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition and measured every 30 se...More data for this Ligand-Target Pair
Affinity DataIC50: 91nMAssay Description:Inhibition of human BChE using butyrylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition and measured every 30 se...More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Inhibition of human BChE using butyrylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition and measured every 30 se...More data for this Ligand-Target Pair
Affinity DataIC50: 126nMAssay Description:Inhibition of human BChE using butyrylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition and measured every 30 se...More data for this Ligand-Target Pair
Affinity DataIC50: 138nMAssay Description:The enzyme activities were determined by measuring the release of fluorescent 6,8-difluoro-4-methylumbelliferone (DiFMU) by the APT hydrolysis of DiF...More data for this Ligand-Target Pair
Affinity DataIC50: 139nMAssay Description:Inhibition of human BChE using butyrylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition and measured every 30 se...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Julius Maximilian University Of W�Rzburg
Curated by ChEMBL
Julius Maximilian University Of W�Rzburg
Curated by ChEMBL
Affinity DataIC50: 175nMAssay Description:Inhibition of human AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and measured every 30 se...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Julius Maximilian University Of W�Rzburg
Curated by ChEMBL
Julius Maximilian University Of W�Rzburg
Curated by ChEMBL
Affinity DataIC50: 175nMAssay Description:Inhibition of human AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and measured every 30 se...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Julius Maximilian University Of W�Rzburg
Curated by ChEMBL
Julius Maximilian University Of W�Rzburg
Curated by ChEMBL
Affinity DataIC50: 188nMAssay Description:Inhibition of human AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and measured every 30 se...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Julius Maximilian University Of W�Rzburg
Curated by ChEMBL
Julius Maximilian University Of W�Rzburg
Curated by ChEMBL
Affinity DataIC50: 200nMAssay Description:Inhibition of human AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and measured every 30 se...More data for this Ligand-Target Pair
Affinity DataIC50: 210nMAssay Description:Binding affinity to human recombinant carbonyl reductase 1 expressed in Escherichia coli assessed as NADPH oxidation using isatin as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 238nMAssay Description:The enzyme activities were determined by measuring the release of fluorescent 6,8-difluoro-4-methylumbelliferone (DiFMU) by the APT hydrolysis of DiF...More data for this Ligand-Target Pair
Affinity DataIC50: 301nMAssay Description:Pseudo-irreversible inhibition of human BChE using butyrylthiocholineiodide as substrate preincubated for 20 to 60 mins followed by substrate additio...More data for this Ligand-Target Pair
Affinity DataIC50: 400nMAssay Description:Binding affinity to human recombinant carbonyl reductase 1 expressed in Escherichia coli assessed as NADPH oxidation using isatin as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 418nMAssay Description:The enzyme activities were determined by measuring the release of fluorescent 6,8-difluoro-4-methylumbelliferone (DiFMU) by the APT hydrolysis of DiF...More data for this Ligand-Target Pair
Affinity DataIC50: 510nMAssay Description:The enzyme activities were determined by measuring the release of fluorescent 6,8-difluoro-4-methylumbelliferone (DiFMU) by the APT hydrolysis of DiF...More data for this Ligand-Target Pair
Affinity DataIC50: 529nMAssay Description:The enzyme activities were determined by measuring the release of fluorescent 6,8-difluoro-4-methylumbelliferone (DiFMU) by the APT hydrolysis of DiF...More data for this Ligand-Target Pair
Affinity DataIC50: 670nMAssay Description:Binding affinity to human recombinant carbonyl reductase 1 expressed in Escherichia coli assessed as NADPH oxidation using isatin as substrateMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Julius Maximilian University Of W�Rzburg
Curated by ChEMBL
Julius Maximilian University Of W�Rzburg
Curated by ChEMBL
Affinity DataIC50: 711nMAssay Description:Inhibition of human AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and measured every 30 se...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Julius Maximilian University Of W�Rzburg
Curated by ChEMBL
Julius Maximilian University Of W�Rzburg
Curated by ChEMBL
Affinity DataIC50: 711nMAssay Description:Inhibition of human AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and measured every 30 se...More data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+3nMAssay Description:The enzyme activities were determined by measuring the release of fluorescent 6,8-difluoro-4-methylumbelliferone (DiFMU) by the APT hydrolysis of DiF...More data for this Ligand-Target Pair
Affinity DataIC50: 1.40E+3nMAssay Description:The enzyme activities were determined by measuring the release of fluorescent 6,8-difluoro-4-methylumbelliferone (DiFMU) by the APT hydrolysis of DiF...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Julius Maximilian University Of W�Rzburg
Curated by ChEMBL
Julius Maximilian University Of W�Rzburg
Curated by ChEMBL
Affinity DataIC50: 1.50E+3nMAssay Description:Pseudo-irreversible inhibition of human AChE using acetylthiocholineiodide as substrate preincubated for 20 to 60 mins followed by substrate addition...More data for this Ligand-Target Pair
Affinity DataIC50: 1.60E+3nMAssay Description:The enzyme activities were determined by measuring the release of fluorescent 6,8-difluoro-4-methylumbelliferone (DiFMU) by the APT hydrolysis of DiF...More data for this Ligand-Target Pair
Affinity DataIC50: 1.80E+3nMAssay Description:The enzyme activities were determined by measuring the release of fluorescent 6,8-difluoro-4-methylumbelliferone (DiFMU) by the APT hydrolysis of DiF...More data for this Ligand-Target Pair


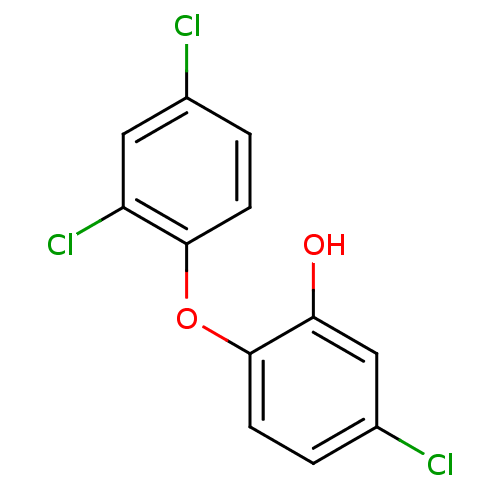
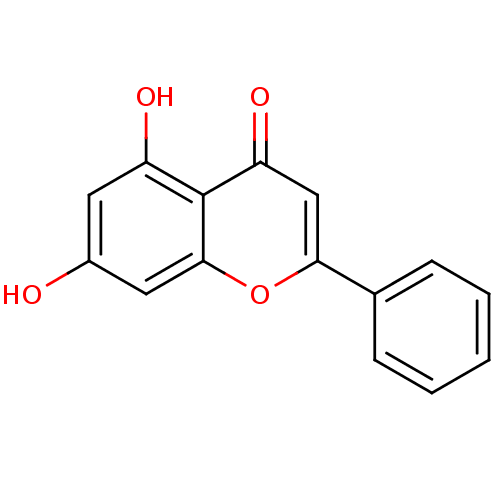


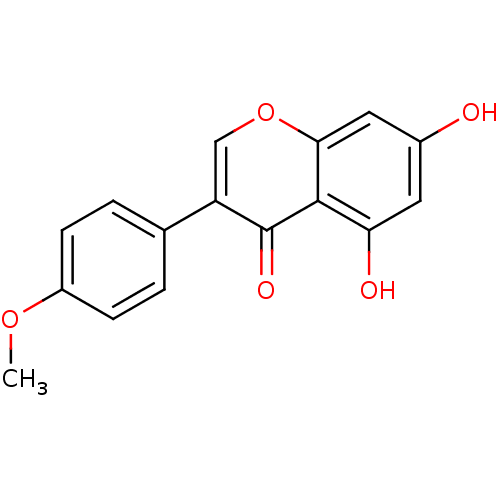




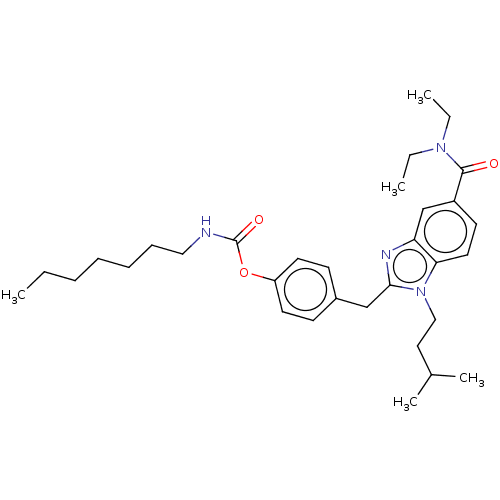


 3D Structure (crystal)
3D Structure (crystal)