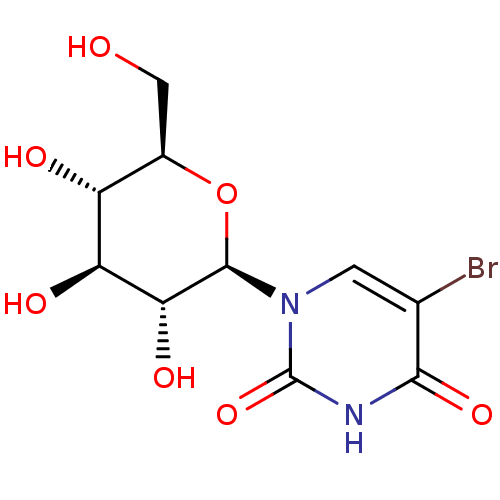
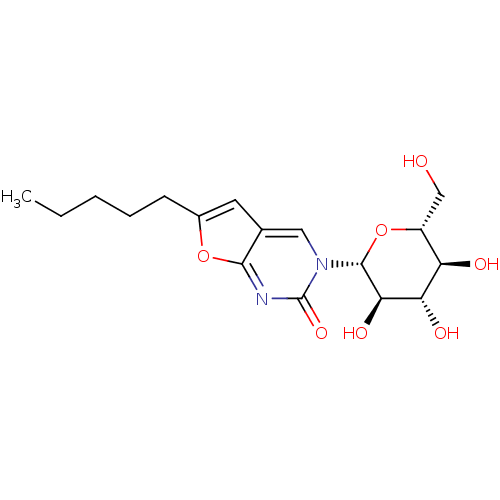
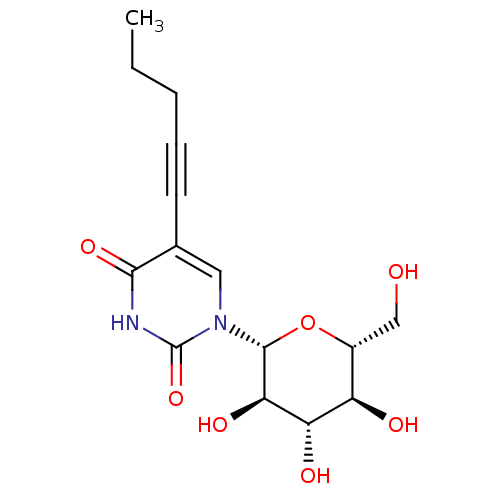
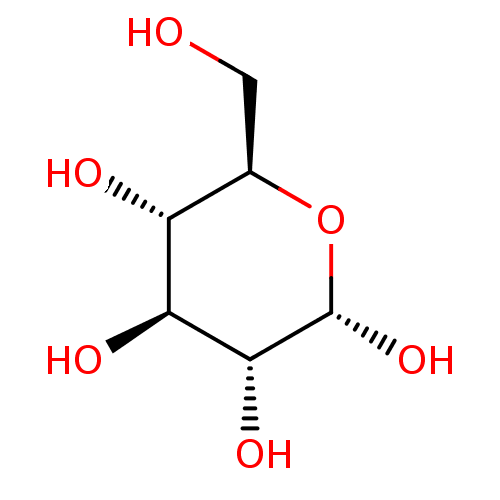
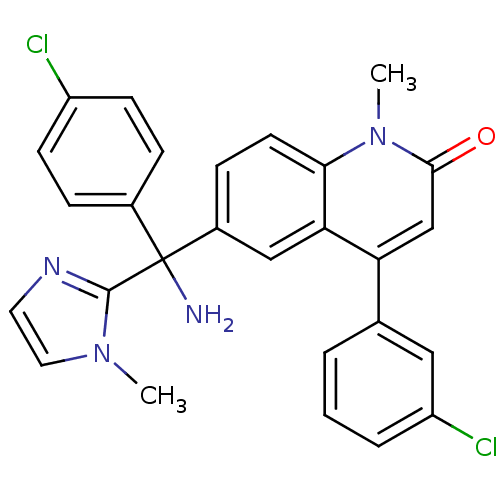
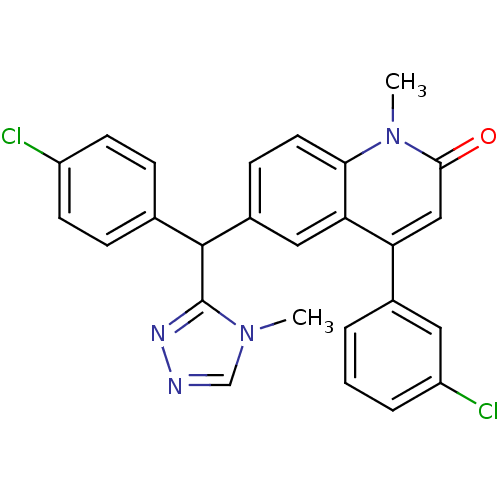
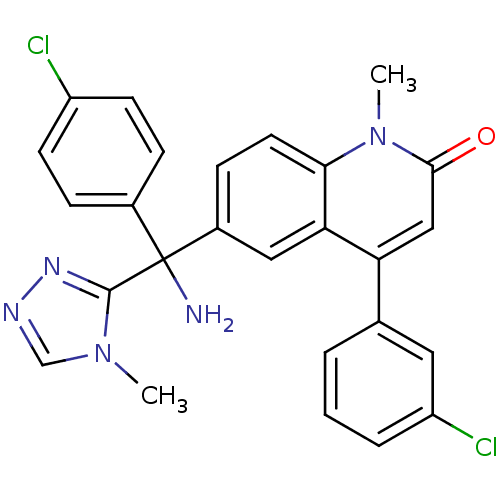
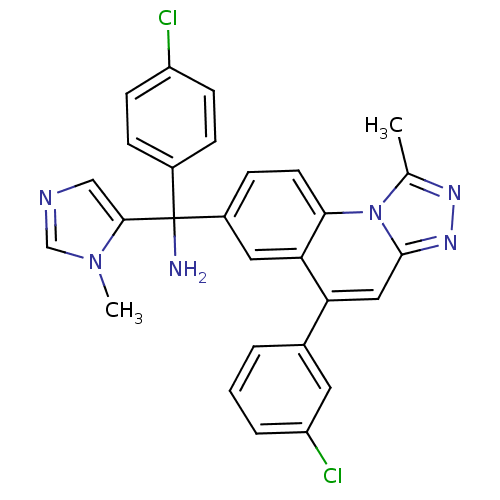
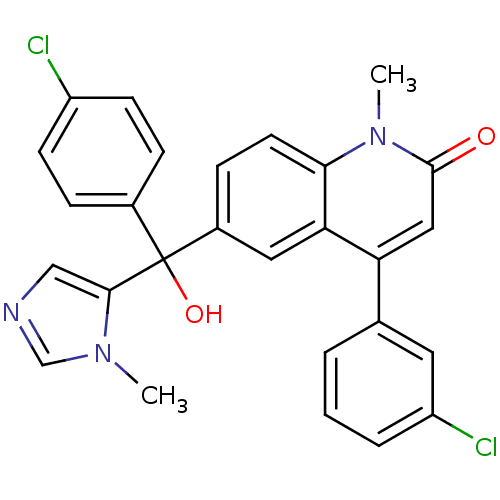
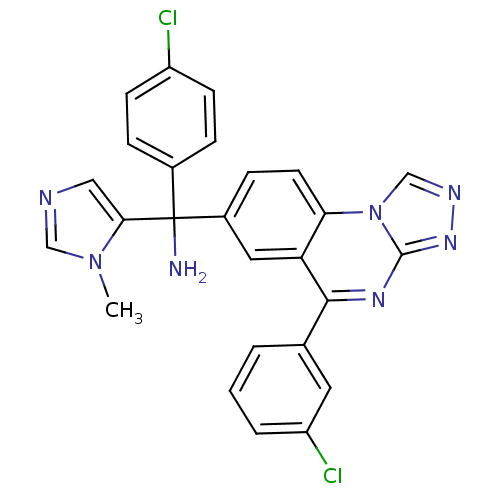
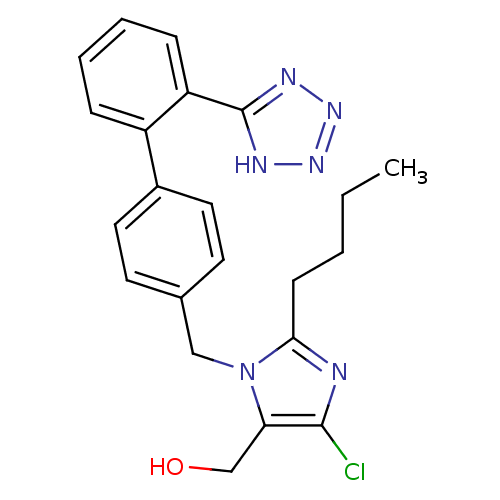
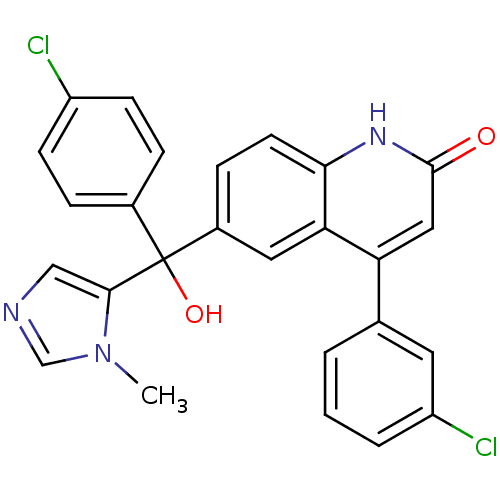
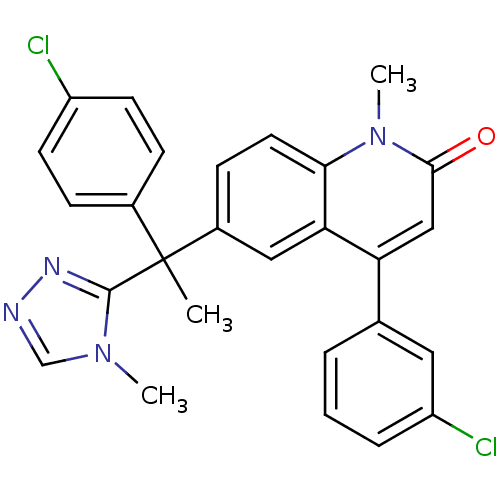
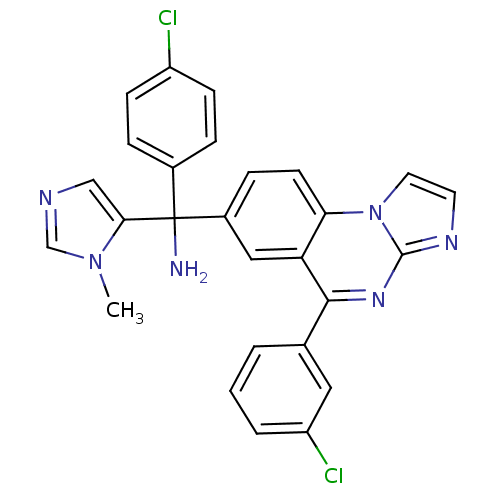
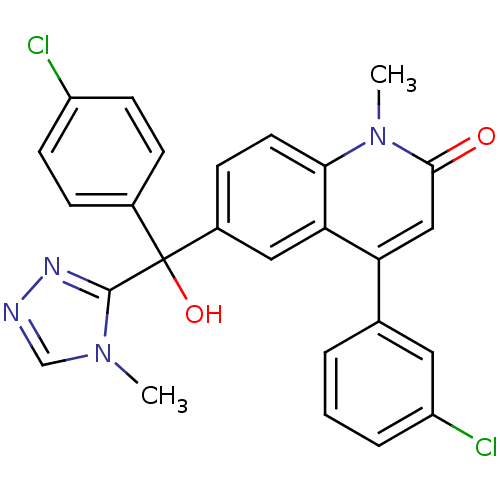
Affinity DataKi: 0.440nMAssay Description:Binding affinity to recombinant human His-tagged MDM2 (1 to 118 amino acids) using p53-based PMDM6-F as probe after 15 mins by competition assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.610nMAssay Description:Binding affinity to recombinant human His-tagged MDM2 (1 to 118 amino acids) using p53-based PMDM6-F as probe after 15 mins by competition assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.620nMAssay Description:Binding affinity to recombinant human His-tagged MDM2 (1 to 118 amino acids) using p53-based PMDM6-F as probe after 15 mins by competition assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.620nMAssay Description:Binding affinity to recombinant human His-tagged MDM2 (1 to 118 amino acids) using p53-based PMDM6-F as probe after 15 mins by competition assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.860nMAssay Description:Binding affinity to recombinant human His-tagged MDM2 (1 to 118 amino acids) using p53-based PMDM6-F as probe after 15 mins by competition assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.940nMAssay Description:Binding affinity to recombinant human His-tagged MDM2 (1 to 118 amino acids) using p53-based PMDM6-F as probe after 15 mins by competition assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.970nMAssay Description:Binding affinity to recombinant human His-tagged MDM2 (1 to 118 amino acids) using p53-based PMDM6-F as probe after 15 mins by competition assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.10nMAssay Description:Binding affinity to recombinant human His-tagged MDM2 (1 to 118 amino acids) using p53-based PMDM6-F as probe after 15 mins by competition assayMore data for this Ligand-Target Pair
Affinity DataKi: 2.40nMAssay Description:Binding affinity to recombinant human His-tagged MDM2 (1 to 118 amino acids) using p53-based PMDM6-F as probe after 15 mins by competition assayMore data for this Ligand-Target Pair
Affinity DataKi: 11nMAssay Description:Binding affinity to recombinant human His-tagged MDM2 (1 to 118 amino acids) using p53-based PMDM6-F as probe after 15 mins by competition assayMore data for this Ligand-Target Pair
Affinity DataKi: 240nMAssay Description:Inhibition of bovine pancreatic RNase AMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, muscle form(Oryctolagus cuniculus (rabbit))
University Of Thessaly
Curated by ChEMBL
University Of Thessaly
Curated by ChEMBL
Affinity DataKi: 1.02E+3nMAssay Description:Competitive inhibition of rabbit skeletal muscle glycogen phosphorylase b using Glc-1-P as substrateMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, muscle form(Homo sapiens (Human))
University Of Thessaly
Curated by ChEMBL
University Of Thessaly
Curated by ChEMBL
Affinity DataKi: 1.02E+3nMAssay Description:Inhibition of glycogen phosphorylase bMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, muscle form(Homo sapiens (Human))
University Of Thessaly
Curated by ChEMBL
University Of Thessaly
Curated by ChEMBL
Affinity DataKi: 1.94E+3nMAssay Description:Inhibition of glycogen phosphorylase bMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, muscle form(Homo sapiens (Human))
University Of Thessaly
Curated by ChEMBL
University Of Thessaly
Curated by ChEMBL
Affinity DataKi: 3.27E+3nMAssay Description:Inhibition of glycogen phosphorylase bMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, muscle form(Oryctolagus cuniculus (rabbit))
University Of Thessaly
Curated by ChEMBL
University Of Thessaly
Curated by ChEMBL
Affinity DataKi: 4.70E+3nMAssay Description:Competitive inhibition of rabbit skeletal muscle glycogen phosphorylase b using Glc-1-P as substrateMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, muscle form(Oryctolagus cuniculus (rabbit))
University Of Thessaly
Curated by ChEMBL
University Of Thessaly
Curated by ChEMBL
Affinity DataKi: 3.24E+4nMAssay Description:Competitive inhibition of rabbit skeletal muscle glycogen phosphorylase b using Glc-1-P as substrateMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, muscle form(Oryctolagus cuniculus (rabbit))
University Of Thessaly
Curated by ChEMBL
University Of Thessaly
Curated by ChEMBL
Affinity DataKi: 3.34E+4nMAssay Description:Competitive inhibition of rabbit skeletal muscle glycogen phosphorylase b using Glc-1-P as substrateMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, muscle form(Oryctolagus cuniculus (rabbit))
University Of Thessaly
Curated by ChEMBL
University Of Thessaly
Curated by ChEMBL
Affinity DataKi: 3.03E+5nMAssay Description:Competitive inhibition of rabbit skeletal muscle glycogen phosphorylase b using Glc-1-P as substrateMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, muscle form(Oryctolagus cuniculus (rabbit))
University Of Thessaly
Curated by ChEMBL
University Of Thessaly
Curated by ChEMBL
Affinity DataKi: 1.70E+6nMAssay Description:Competitive inhibition of rabbit skeletal muscle glycogen phosphorylase b using Glc-1-P as substrateMore data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&Jprd)
Curated by ChEMBL
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&Jprd)
Curated by ChEMBL
Affinity DataIC50: 0.600nMAssay Description:Inhibition of [3H]-FPP incorporation into biotinylated laminB peptide by farnesyl transferaseMore data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&Jprd)
Curated by ChEMBL
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&Jprd)
Curated by ChEMBL
Affinity DataIC50: 0.600nMAssay Description:Inhibition of Geranylgeranylprotein transferase-I catalyzed incorporation of [3H]-GGPP into biotinYRASNRSCAIL peptideMore data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&Jprd)
Curated by ChEMBL
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&Jprd)
Curated by ChEMBL
Affinity DataIC50: 0.900nMAssay Description:Inhibition of [3H]-FPP incorporation into biotinylated lamin B peptide by farnesyl transferaseMore data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&Jprd)
Curated by ChEMBL
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&Jprd)
Curated by ChEMBL
Affinity DataIC50: 0.900nMAssay Description:Inhibition of [3H]-FPP incorporation into biotinylated laminB peptide by farnesyl transferaseMore data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&Jprd)
Curated by ChEMBL
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&Jprd)
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of [3H]-FPP incorporation into biotinylated lamin B peptide by farnesyl transferaseMore data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&Jprd)
Curated by ChEMBL
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&Jprd)
Curated by ChEMBL
Affinity DataIC50: 1.30nMAssay Description:Inhibition of [3H]-FPP incorporation into biotinylated lamin B peptide by farnesyl transferaseMore data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&Jprd)
Curated by ChEMBL
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&Jprd)
Curated by ChEMBL
Affinity DataIC50: 1.5nMAssay Description:Inhibition of [3H]-FPP incorporation into biotinylated laminB peptide by farnesyl transferaseMore data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&Jprd)
Curated by ChEMBL
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&Jprd)
Curated by ChEMBL
Affinity DataIC50: 1.70nMAssay Description:Inhibition of Geranylgeranylprotein transferase-I catalyzed incorporation of [3H]-GGPP into biotinYRASNRSCAIL peptideMore data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&Jprd)
Curated by ChEMBL
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&Jprd)
Curated by ChEMBL
Affinity DataIC50: 1.70nMAssay Description:Inhibition of [3H]-FPP incorporation into biotinylated laminB peptide by farnesyl transferaseMore data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&Jprd)
Curated by ChEMBL
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&Jprd)
Curated by ChEMBL
Affinity DataIC50: 1.70nMAssay Description:Inhibition of [3H]-FPP incorporation into biotinylated lamin B peptide by farnesyl transferaseMore data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&Jprd)
Curated by ChEMBL
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&Jprd)
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of [3H]-FPP incorporation into biotinylated laminB peptide by farnesyl transferaseMore data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&Jprd)
Curated by ChEMBL
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&Jprd)
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of [3H]-FPP incorporation into biotinylated lamin B peptide by farnesyl transferaseMore data for this Ligand-Target Pair
TargetType-1 angiotensin II receptor(Homo sapiens (Human))
National Hellenic Research Foundation
Curated by ChEMBL
National Hellenic Research Foundation
Curated by ChEMBL
Affinity DataIC50: 3.20nMAssay Description:Displacement of [125I-Sar1-Ile8]Ang2 from human AT1 receptor expressed in HEK293 cell membrane incubated for 1 hr by gamma counting methodMore data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&Jprd)
Curated by ChEMBL
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&Jprd)
Curated by ChEMBL
Affinity DataIC50: 3.5nMAssay Description:Inhibition of Geranylgeranylprotein transferase-I catalyzed incorporation of [3H]-GGPP into biotinYRASNRSCAIL peptideMore data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&Jprd)
Curated by ChEMBL
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&Jprd)
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of [3H]-FPP incorporation into biotinylated lamin B peptide by farnesyl transferaseMore data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&Jprd)
Curated by ChEMBL
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&Jprd)
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of [3H]-FPP incorporation into biotinylated lamin B peptide by farnesyl transferaseMore data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&Jprd)
Curated by ChEMBL
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&Jprd)
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of [3H]-FPP incorporation into biotinylated lamin B peptide by farnesyl transferaseMore data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&Jprd)
Curated by ChEMBL
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&Jprd)
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of [3H]-FPP incorporation into biotinylated laminB peptide by farnesyl transferaseMore data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&Jprd)
Curated by ChEMBL
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&Jprd)
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Inhibition of [3H]-FPP incorporation into biotinylated lamin B peptide by farnesyl transferaseMore data for this Ligand-Target Pair
Affinity DataIC50: 6.80nMAssay Description:Binding affinity to recombinant human His-tagged MDM2 (1 to 118 amino acids) using p53-based PMDM6-F as probe after 15 to 30 mins by fluorescence pol...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&Jprd)
Curated by ChEMBL
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&Jprd)
Curated by ChEMBL
Affinity DataIC50: 7nMAssay Description:Inhibition of [3H]-FPP incorporation into biotinylated laminB peptide by farnesyl transferaseMore data for this Ligand-Target Pair
Affinity DataIC50: 7.10nMAssay Description:Binding affinity to recombinant human His-tagged MDM2 (1 to 118 amino acids) using p53-based PMDM6-F as probe after 15 to 30 mins by fluorescence pol...More data for this Ligand-Target Pair
Affinity DataIC50: 7.60nMAssay Description:Binding affinity to recombinant human His-tagged MDM2 (1 to 118 amino acids) using p53-based PMDM6-F as probe after 15 to 30 mins by fluorescence pol...More data for this Ligand-Target Pair
Affinity DataIC50: 8.20nMAssay Description:Binding affinity to recombinant human His-tagged MDM2 (1 to 118 amino acids) using p53-based PMDM6-F as probe after 15 to 30 mins by fluorescence pol...More data for this Ligand-Target Pair
Affinity DataIC50: 8.40nMAssay Description:Binding affinity to recombinant human His-tagged MDM2 (1 to 118 amino acids) using p53-based PMDM6-F as probe after 15 to 30 mins by fluorescence pol...More data for this Ligand-Target Pair
Affinity DataIC50: 8.80nMAssay Description:Binding affinity to recombinant human His-tagged MDM2 (1 to 118 amino acids) using p53-based PMDM6-F as probe after 15 to 30 mins by fluorescence pol...More data for this Ligand-Target Pair
Affinity DataIC50: 9.80nMAssay Description:Binding affinity to recombinant human His-tagged MDM2 (1 to 118 amino acids) using p53-based PMDM6-F as probe after 15 to 30 mins by fluorescence pol...More data for this Ligand-Target Pair




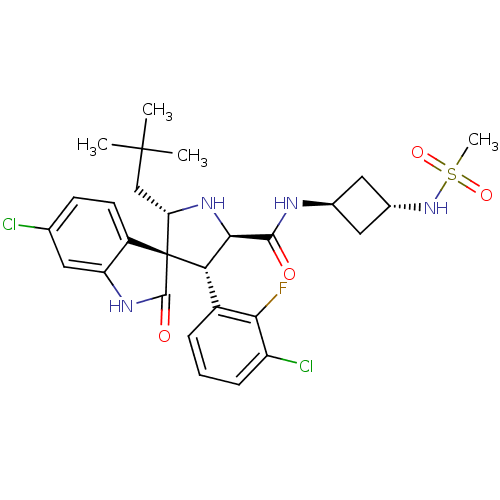
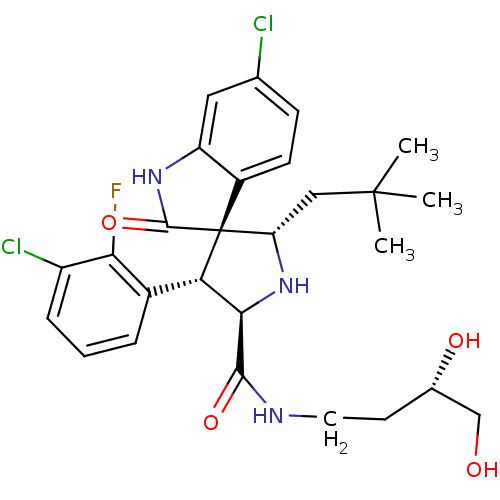


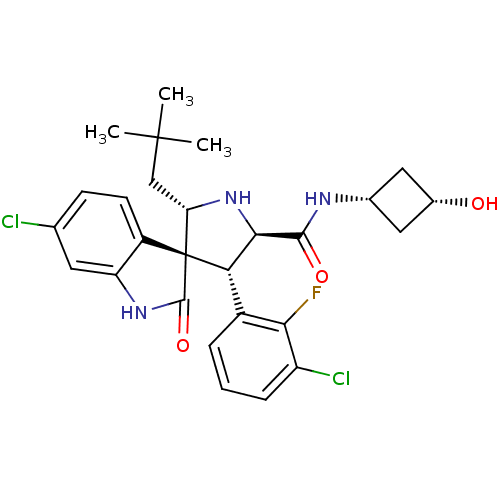
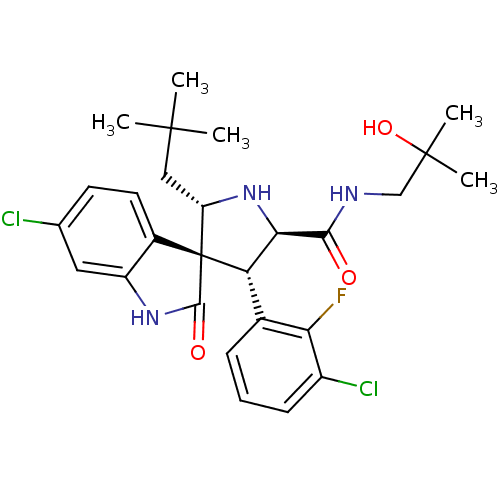
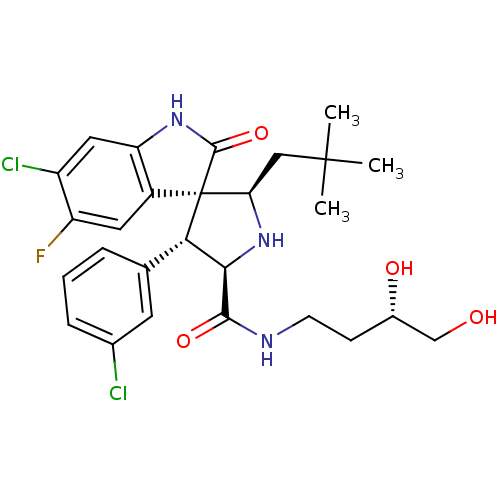



 3D Structure (crystal)
3D Structure (crystal)