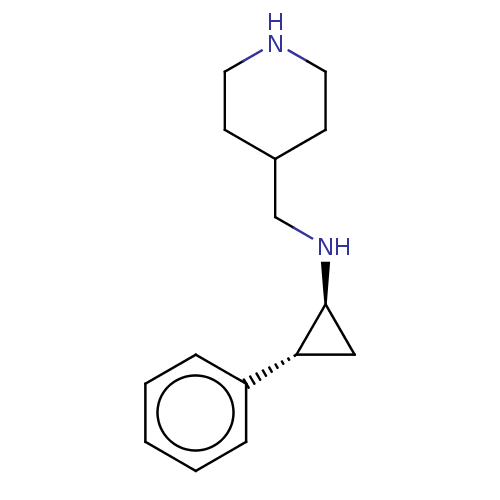
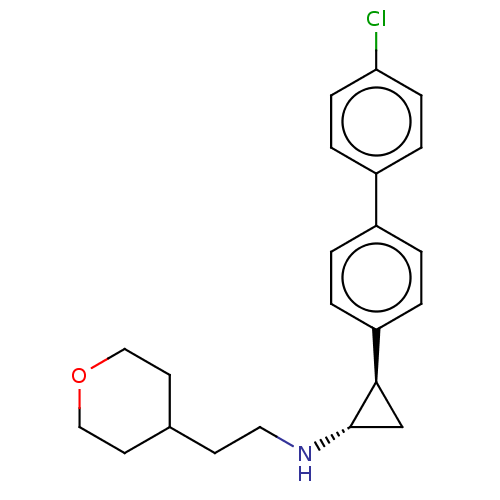
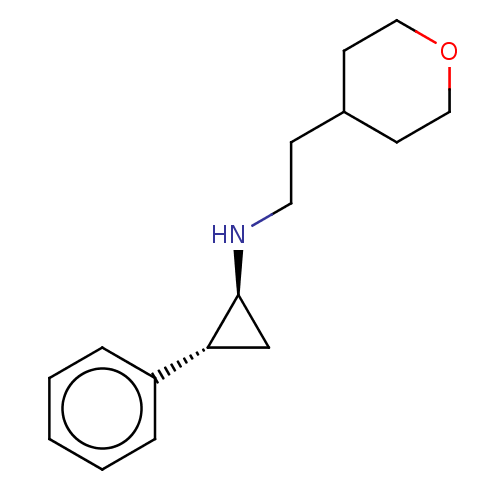
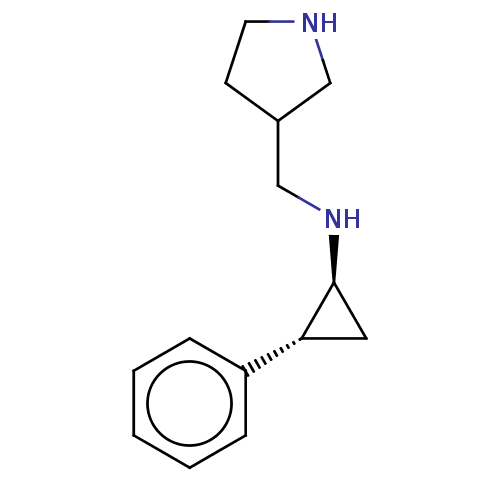
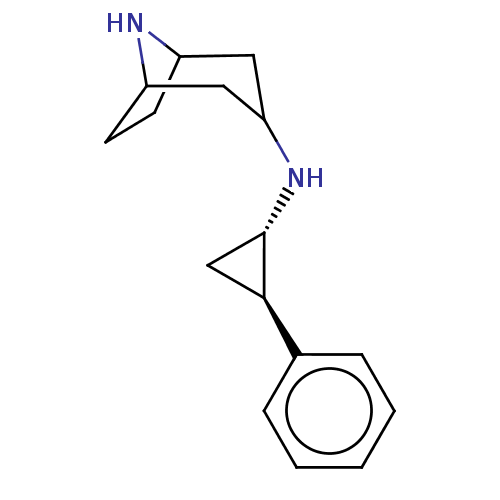
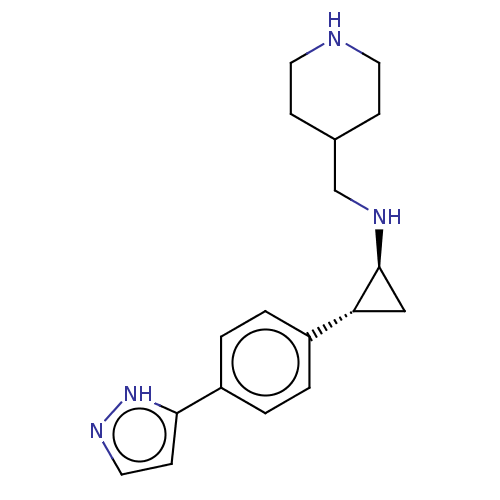
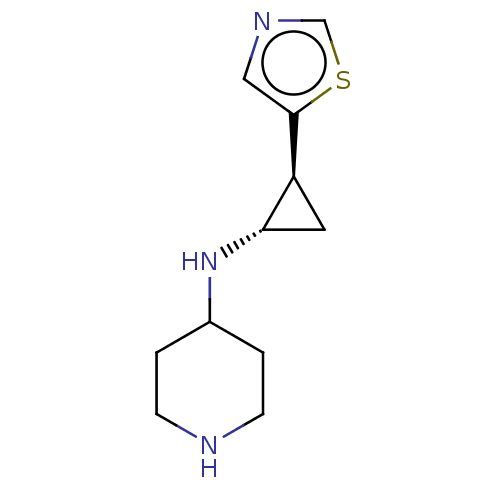
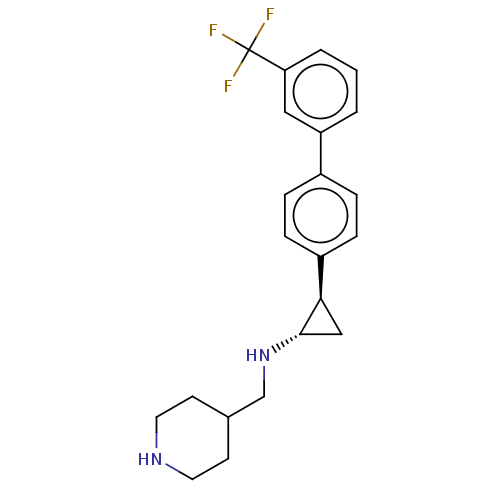
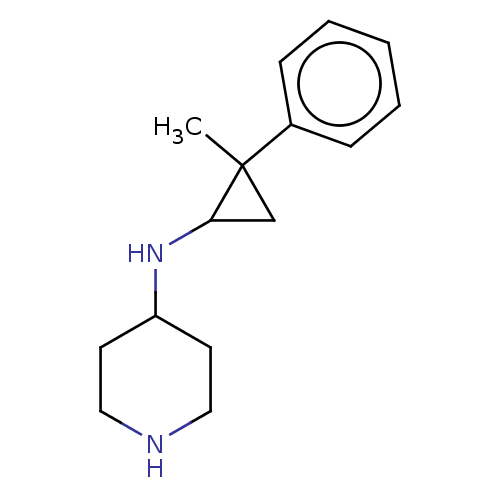
TargetGlutathione S-transferase P/Isoform 2 of Lysine-specific histone demethylase 1A (2) [158-876](Homo sapiens (Human))
Oryzon Genomics
US Patent
Oryzon Genomics
US Patent
Affinity DataKi: 50nM ΔG°: -43.3kJ/molepH: 7.4 T: 2°CAssay Description:The compounds of the invention can be tested for their ability to inhibit LSD1. The ability of the compounds of the invention to inhibit LSD1 can be ...More data for this Ligand-Target Pair
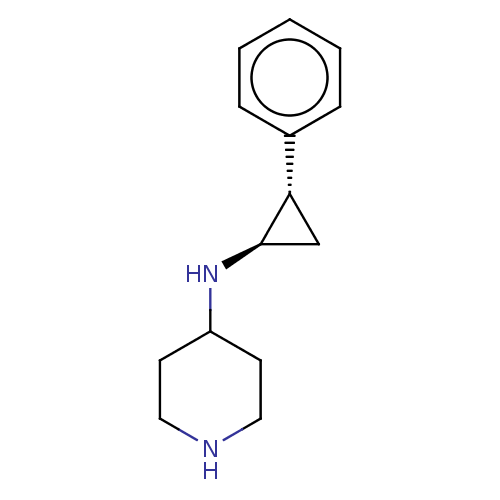
TargetGlutathione S-transferase P/Isoform 2 of Lysine-specific histone demethylase 1A (2) [158-876](Homo sapiens (Human))
Oryzon Genomics
US Patent
Oryzon Genomics
US Patent
Affinity DataKi: 50nM ΔG°: -43.3kJ/molepH: 7.4 T: 2°CAssay Description:The compounds of the invention can be tested for their ability to inhibit LSD1. The ability of the compounds of the invention to inhibit LSD1 can be ...More data for this Ligand-Target Pair
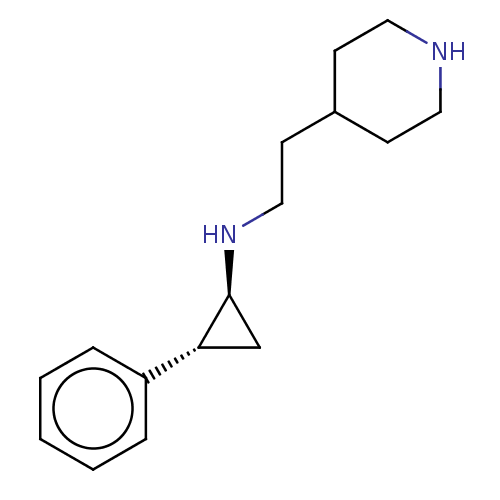
TargetGlutathione S-transferase P/Isoform 2 of Lysine-specific histone demethylase 1A (2) [158-876](Homo sapiens (Human))
Oryzon Genomics
US Patent
Oryzon Genomics
US Patent
Affinity DataKi: 50nM ΔG°: -43.3kJ/molepH: 7.4 T: 2°CAssay Description:The compounds of the invention can be tested for their ability to inhibit LSD1. The ability of the compounds of the invention to inhibit LSD1 can be ...More data for this Ligand-Target Pair
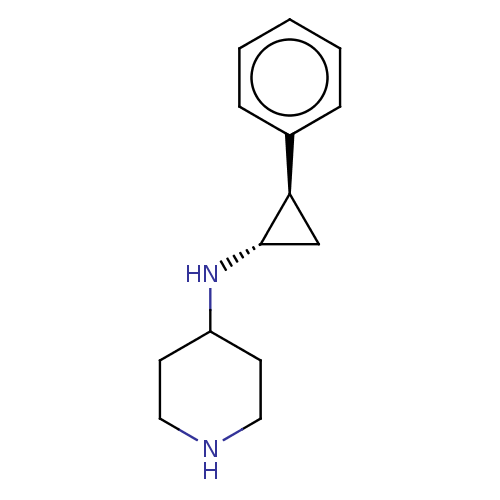
TargetGlutathione S-transferase P/Isoform 2 of Lysine-specific histone demethylase 1A (2) [158-876](Homo sapiens (Human))
Oryzon Genomics
US Patent
Oryzon Genomics
US Patent
Affinity DataKi: 50nM ΔG°: -43.3kJ/molepH: 7.4 T: 2°CAssay Description:The compounds of the invention can be tested for their ability to inhibit LSD1. The ability of the compounds of the invention to inhibit LSD1 can be ...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Isoform 2 of Lysine-specific histone demethylase 1A (2) [158-876](Homo sapiens (Human))
Oryzon Genomics
US Patent
Oryzon Genomics
US Patent
Affinity DataKi: 50nM ΔG°: -43.3kJ/molepH: 7.4 T: 2°CAssay Description:The compounds of the invention can be tested for their ability to inhibit LSD1. The ability of the compounds of the invention to inhibit LSD1 can be ...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Isoform 2 of Lysine-specific histone demethylase 1A (2) [158-876](Homo sapiens (Human))
Oryzon Genomics
US Patent
Oryzon Genomics
US Patent
Affinity DataKi: 550nM ΔG°: -37.2kJ/molepH: 7.4 T: 2°CAssay Description:The compounds of the invention can be tested for their ability to inhibit LSD1. The ability of the compounds of the invention to inhibit LSD1 can be ...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Isoform 2 of Lysine-specific histone demethylase 1A (2) [158-876](Homo sapiens (Human))
Oryzon Genomics
US Patent
Oryzon Genomics
US Patent
Affinity DataKi: 550nM ΔG°: -37.2kJ/molepH: 7.4 T: 2°CAssay Description:The compounds of the invention can be tested for their ability to inhibit LSD1. The ability of the compounds of the invention to inhibit LSD1 can be ...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Isoform 2 of Lysine-specific histone demethylase 1A (2) [158-876](Homo sapiens (Human))
Oryzon Genomics
US Patent
Oryzon Genomics
US Patent
Affinity DataKi: 550nM ΔG°: -37.2kJ/molepH: 7.4 T: 2°CAssay Description:The compounds of the invention can be tested for their ability to inhibit LSD1. The ability of the compounds of the invention to inhibit LSD1 can be ...More data for this Ligand-Target Pair
Affinity DataKi: 5.50E+3nM ΔG°: -31.2kJ/molepH: 7.5 T: 2°CAssay Description:Human recombinant monoamine oxidase proteins MAO-A and MAO-B were purchased from Sigma Aldrich. MAOs catalyze the oxidative deamination of primary, s...More data for this Ligand-Target Pair
Affinity DataKi: 5.50E+3nM ΔG°: -31.2kJ/molepH: 7.5 T: 2°CAssay Description:Human recombinant monoamine oxidase proteins MAO-A and MAO-B were purchased from Sigma Aldrich. MAOs catalyze the oxidative deamination of primary, s...More data for this Ligand-Target Pair
Affinity DataKi: 5.50E+3nM ΔG°: -31.2kJ/molepH: 7.5 T: 2°CAssay Description:Human recombinant monoamine oxidase proteins MAO-A and MAO-B were purchased from Sigma Aldrich. MAOs catalyze the oxidative deamination of primary, s...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Isoform 2 of Lysine-specific histone demethylase 1A (2) [158-876](Homo sapiens (Human))
Oryzon Genomics
US Patent
Oryzon Genomics
US Patent
Affinity DataKi: 5.50E+3nM ΔG°: -31.2kJ/molepH: 7.4 T: 2°CAssay Description:The compounds of the invention can be tested for their ability to inhibit LSD1. The ability of the compounds of the invention to inhibit LSD1 can be ...More data for this Ligand-Target Pair
Affinity DataKi: 5.50E+4nM ΔG°: -25.3kJ/molepH: 7.5 T: 2°CAssay Description:Human recombinant monoamine oxidase proteins MAO-A and MAO-B were purchased from Sigma Aldrich. MAOs catalyze the oxidative deamination of primary, s...More data for this Ligand-Target Pair
Affinity DataKi: 5.50E+4nM ΔG°: -25.3kJ/molepH: 7.5 T: 2°CAssay Description:Human recombinant monoamine oxidase proteins MAO-A and MAO-B were purchased from Sigma Aldrich. MAOs catalyze the oxidative deamination of primary, s...More data for this Ligand-Target Pair
Affinity DataKi: 5.50E+4nM ΔG°: -25.3kJ/molepH: 7.5 T: 2°CAssay Description:Human recombinant monoamine oxidase proteins MAO-A and MAO-B were purchased from Sigma Aldrich. MAOs catalyze the oxidative deamination of primary, s...More data for this Ligand-Target Pair
Affinity DataKi: 5.50E+4nM ΔG°: -25.3kJ/molepH: 7.5 T: 2°CAssay Description:Human recombinant monoamine oxidase proteins MAO-A and MAO-B were purchased from Sigma Aldrich. MAOs catalyze the oxidative deamination of primary, s...More data for this Ligand-Target Pair
Affinity DataKi: 5.50E+4nM ΔG°: -25.3kJ/molepH: 7.5 T: 2°CAssay Description:Human recombinant monoamine oxidase proteins MAO-A and MAO-B were purchased from Sigma Aldrich. MAOs catalyze the oxidative deamination of primary, s...More data for this Ligand-Target Pair
Affinity DataKi: 5.50E+4nM ΔG°: -25.3kJ/molepH: 7.5 T: 2°CAssay Description:Human recombinant monoamine oxidase proteins MAO-A and MAO-B were purchased from Sigma Aldrich. MAOs catalyze the oxidative deamination of primary, s...More data for this Ligand-Target Pair
Affinity DataKi: 5.50E+4nM ΔG°: -25.3kJ/molepH: 7.5 T: 2°CAssay Description:Human recombinant monoamine oxidase proteins MAO-A and MAO-B were purchased from Sigma Aldrich. MAOs catalyze the oxidative deamination of primary, s...More data for this Ligand-Target Pair
Affinity DataKi: 5.50E+4nM ΔG°: -25.3kJ/molepH: 7.5 T: 2°CAssay Description:Human recombinant monoamine oxidase proteins MAO-A and MAO-B were purchased from Sigma Aldrich. MAOs catalyze the oxidative deamination of primary, s...More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nM ΔG°: >-23.7kJ/molepH: 7.5 T: 2°CAssay Description:Human recombinant monoamine oxidase proteins MAO-A and MAO-B were purchased from Sigma Aldrich. MAOs catalyze the oxidative deamination of primary, s...More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nM ΔG°: >-23.7kJ/molepH: 7.5 T: 2°CAssay Description:Human recombinant monoamine oxidase proteins MAO-A and MAO-B were purchased from Sigma Aldrich. MAOs catalyze the oxidative deamination of primary, s...More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nM ΔG°: >-23.7kJ/molepH: 7.5 T: 2°CAssay Description:Human recombinant monoamine oxidase proteins MAO-A and MAO-B were purchased from Sigma Aldrich. MAOs catalyze the oxidative deamination of primary, s...More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nM ΔG°: >-23.7kJ/molepH: 7.5 T: 2°CAssay Description:Human recombinant monoamine oxidase proteins MAO-A and MAO-B were purchased from Sigma Aldrich. MAOs catalyze the oxidative deamination of primary, s...More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nM ΔG°: >-23.7kJ/molepH: 7.5 T: 2°CAssay Description:Human recombinant monoamine oxidase proteins MAO-A and MAO-B were purchased from Sigma Aldrich. MAOs catalyze the oxidative deamination of primary, s...More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nM ΔG°: >-23.7kJ/molepH: 7.5 T: 2°CAssay Description:Human recombinant monoamine oxidase proteins MAO-A and MAO-B were purchased from Sigma Aldrich. MAOs catalyze the oxidative deamination of primary, s...More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nM ΔG°: >-23.7kJ/molepH: 7.5 T: 2°CAssay Description:Human recombinant monoamine oxidase proteins MAO-A and MAO-B were purchased from Sigma Aldrich. MAOs catalyze the oxidative deamination of primary, s...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Isoform 2 of Lysine-specific histone demethylase 1A (2) [158-876](Homo sapiens (Human))
Oryzon Genomics
US Patent
Oryzon Genomics
US Patent
Affinity DataIC50: 8nMpH: 7.4 T: 2°CAssay Description:The compounds of the invention can be tested for their ability to inhibit LSD1. The ability of the compounds of the invention to inhibit LSD1 can be ...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Isoform 2 of Lysine-specific histone demethylase 1A (2) [158-876](Homo sapiens (Human))
Oryzon Genomics
US Patent
Oryzon Genomics
US Patent
Affinity DataIC50: 19nMpH: 7.4 T: 2°CAssay Description:The compounds of the invention can be tested for their ability to inhibit LSD1. The ability of the compounds of the invention to inhibit LSD1 can be ...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Isoform 2 of Lysine-specific histone demethylase 1A (2) [158-876](Homo sapiens (Human))
Oryzon Genomics
US Patent
Oryzon Genomics
US Patent
Affinity DataIC50: 22nMpH: 7.4 T: 2°CAssay Description:The compounds of the invention can be tested for their ability to inhibit LSD1. The ability of the compounds of the invention to inhibit LSD1 can be ...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Isoform 2 of Lysine-specific histone demethylase 1A (2) [158-876](Homo sapiens (Human))
Oryzon Genomics
US Patent
Oryzon Genomics
US Patent
Affinity DataIC50: 23nMpH: 7.4 T: 2°CAssay Description:The compounds of the invention can be tested for their ability to inhibit LSD1. The ability of the compounds of the invention to inhibit LSD1 can be ...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Isoform 2 of Lysine-specific histone demethylase 1A (2) [158-876](Homo sapiens (Human))
Oryzon Genomics
US Patent
Oryzon Genomics
US Patent
Affinity DataIC50: 32nMpH: 7.4 T: 2°CAssay Description:The compounds of the invention can be tested for their ability to inhibit LSD1. The ability of the compounds of the invention to inhibit LSD1 can be ...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Isoform 2 of Lysine-specific histone demethylase 1A (2) [158-876](Homo sapiens (Human))
Oryzon Genomics
US Patent
Oryzon Genomics
US Patent
Affinity DataIC50: 35nMpH: 7.4 T: 2°CAssay Description:The compounds of the invention can be tested for their ability to inhibit LSD1. The ability of the compounds of the invention to inhibit LSD1 can be ...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Isoform 2 of Lysine-specific histone demethylase 1A (2) [158-876](Homo sapiens (Human))
Oryzon Genomics
US Patent
Oryzon Genomics
US Patent
Affinity DataIC50: 37nMpH: 7.4 T: 2°CAssay Description:The compounds of the invention can be tested for their ability to inhibit LSD1. The ability of the compounds of the invention to inhibit LSD1 can be ...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Isoform 2 of Lysine-specific histone demethylase 1A (2) [158-876](Homo sapiens (Human))
Oryzon Genomics
US Patent
Oryzon Genomics
US Patent
Affinity DataIC50: 38nMpH: 7.4 T: 2°CAssay Description:The compounds of the invention can be tested for their ability to inhibit LSD1. The ability of the compounds of the invention to inhibit LSD1 can be ...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Isoform 2 of Lysine-specific histone demethylase 1A (2) [158-876](Homo sapiens (Human))
Oryzon Genomics
US Patent
Oryzon Genomics
US Patent
Affinity DataIC50: 38nMpH: 7.4 T: 2°CAssay Description:The compounds of the invention can be tested for their ability to inhibit LSD1. The ability of the compounds of the invention to inhibit LSD1 can be ...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Isoform 2 of Lysine-specific histone demethylase 1A (2) [158-876](Homo sapiens (Human))
Oryzon Genomics
US Patent
Oryzon Genomics
US Patent
Affinity DataIC50: 42nMpH: 7.4 T: 2°CAssay Description:The compounds of the invention can be tested for their ability to inhibit LSD1. The ability of the compounds of the invention to inhibit LSD1 can be ...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Isoform 2 of Lysine-specific histone demethylase 1A (2) [158-876](Homo sapiens (Human))
Oryzon Genomics
US Patent
Oryzon Genomics
US Patent
Affinity DataIC50: 42nMpH: 7.4 T: 2°CAssay Description:The compounds of the invention can be tested for their ability to inhibit LSD1. The ability of the compounds of the invention to inhibit LSD1 can be ...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Isoform 2 of Lysine-specific histone demethylase 1A (2) [158-876](Homo sapiens (Human))
Oryzon Genomics
US Patent
Oryzon Genomics
US Patent
Affinity DataIC50: 47nMpH: 7.4 T: 2°CAssay Description:The compounds of the invention can be tested for their ability to inhibit LSD1. The ability of the compounds of the invention to inhibit LSD1 can be ...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Isoform 2 of Lysine-specific histone demethylase 1A (2) [158-876](Homo sapiens (Human))
Oryzon Genomics
US Patent
Oryzon Genomics
US Patent
Affinity DataIC50: 48nMpH: 7.4 T: 2°CAssay Description:The compounds of the invention can be tested for their ability to inhibit LSD1. The ability of the compounds of the invention to inhibit LSD1 can be ...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Isoform 2 of Lysine-specific histone demethylase 1A (2) [158-876](Homo sapiens (Human))
Oryzon Genomics
US Patent
Oryzon Genomics
US Patent
Affinity DataIC50: 56nMpH: 7.4 T: 2°CAssay Description:The compounds of the invention can be tested for their ability to inhibit LSD1. The ability of the compounds of the invention to inhibit LSD1 can be ...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Isoform 2 of Lysine-specific histone demethylase 1A (2) [158-876](Homo sapiens (Human))
Oryzon Genomics
US Patent
Oryzon Genomics
US Patent
Affinity DataIC50: 68nMpH: 7.4 T: 2°CAssay Description:The compounds of the invention can be tested for their ability to inhibit LSD1. The ability of the compounds of the invention to inhibit LSD1 can be ...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Isoform 2 of Lysine-specific histone demethylase 1A (2) [158-876](Homo sapiens (Human))
Oryzon Genomics
US Patent
Oryzon Genomics
US Patent
Affinity DataIC50: 69nMpH: 7.4 T: 2°CAssay Description:The compounds of the invention can be tested for their ability to inhibit LSD1. The ability of the compounds of the invention to inhibit LSD1 can be ...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Isoform 2 of Lysine-specific histone demethylase 1A (2) [158-876](Homo sapiens (Human))
Oryzon Genomics
US Patent
Oryzon Genomics
US Patent
Affinity DataIC50: 71nMpH: 7.4 T: 2°CAssay Description:The compounds of the invention can be tested for their ability to inhibit LSD1. The ability of the compounds of the invention to inhibit LSD1 can be ...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Isoform 2 of Lysine-specific histone demethylase 1A (2) [158-876](Homo sapiens (Human))
Oryzon Genomics
US Patent
Oryzon Genomics
US Patent
Affinity DataIC50: 72nMpH: 7.4 T: 2°CAssay Description:The compounds of the invention can be tested for their ability to inhibit LSD1. The ability of the compounds of the invention to inhibit LSD1 can be ...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Isoform 2 of Lysine-specific histone demethylase 1A (2) [158-876](Homo sapiens (Human))
Oryzon Genomics
US Patent
Oryzon Genomics
US Patent
Affinity DataIC50: 81nMpH: 7.4 T: 2°CAssay Description:The compounds of the invention can be tested for their ability to inhibit LSD1. The ability of the compounds of the invention to inhibit LSD1 can be ...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Isoform 2 of Lysine-specific histone demethylase 1A (2) [158-876](Homo sapiens (Human))
Oryzon Genomics
US Patent
Oryzon Genomics
US Patent
Affinity DataIC50: 84nMpH: 7.4 T: 2°CAssay Description:The compounds of the invention can be tested for their ability to inhibit LSD1. The ability of the compounds of the invention to inhibit LSD1 can be ...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Isoform 2 of Lysine-specific histone demethylase 1A (2) [158-876](Homo sapiens (Human))
Oryzon Genomics
US Patent
Oryzon Genomics
US Patent
Affinity DataIC50: 97nMpH: 7.4 T: 2°CAssay Description:The compounds of the invention can be tested for their ability to inhibit LSD1. The ability of the compounds of the invention to inhibit LSD1 can be ...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Isoform 2 of Lysine-specific histone demethylase 1A (2) [158-876](Homo sapiens (Human))
Oryzon Genomics
US Patent
Oryzon Genomics
US Patent
Affinity DataIC50: 104nMpH: 7.4 T: 2°CAssay Description:The compounds of the invention can be tested for their ability to inhibit LSD1. The ability of the compounds of the invention to inhibit LSD1 can be ...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Isoform 2 of Lysine-specific histone demethylase 1A (2) [158-876](Homo sapiens (Human))
Oryzon Genomics
US Patent
Oryzon Genomics
US Patent
Affinity DataIC50: 105nMpH: 7.4 T: 2°CAssay Description:The compounds of the invention can be tested for their ability to inhibit LSD1. The ability of the compounds of the invention to inhibit LSD1 can be ...More data for this Ligand-Target Pair