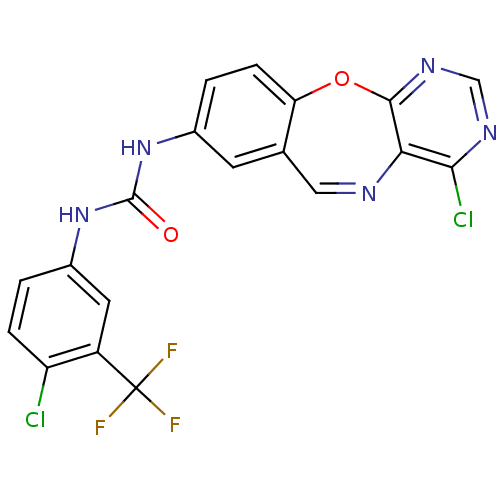
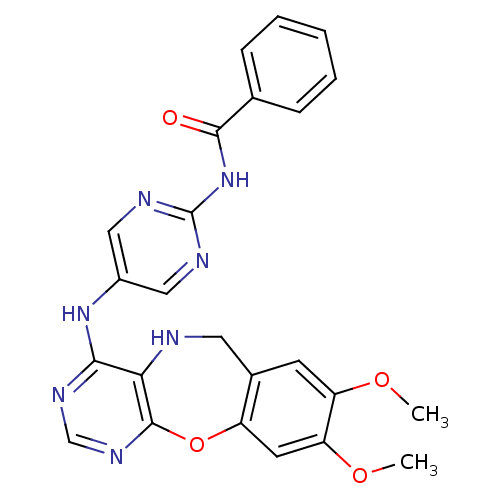
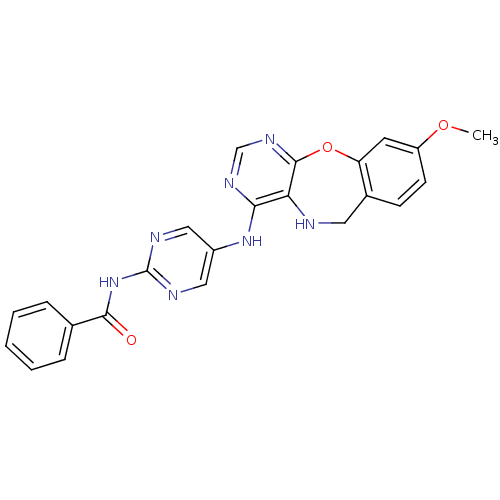
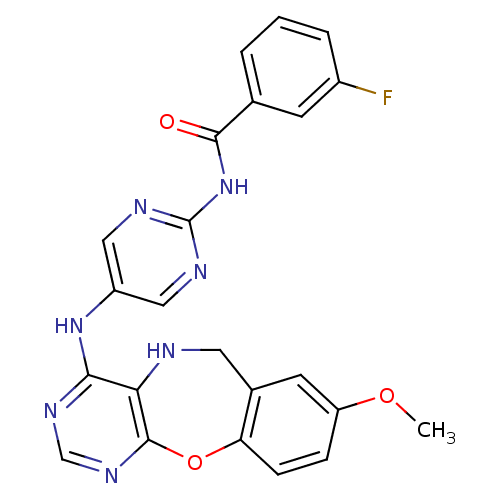
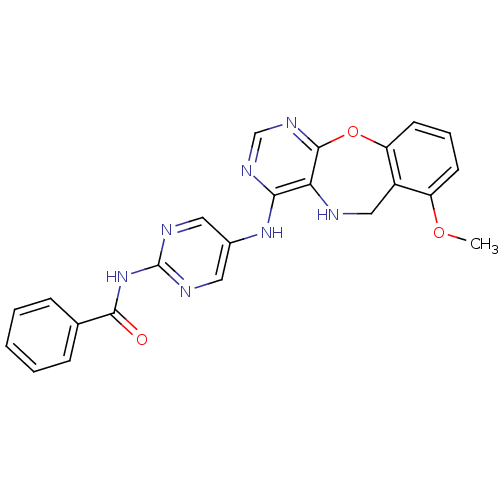
Affinity DataIC50: 320nMAssay Description:Inhibitory activity against Aurora A kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 330nMAssay Description:Inhibitory activity against Aurora A kinaseMore data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein kinase FLT3(Homo sapiens (Human))
Imclone Systems
Curated by ChEMBL
Imclone Systems
Curated by ChEMBL
Affinity DataIC50: 450nMAssay Description:Inhibitory activity against FLT3 kinase in a HTRF assayMore data for this Ligand-Target Pair
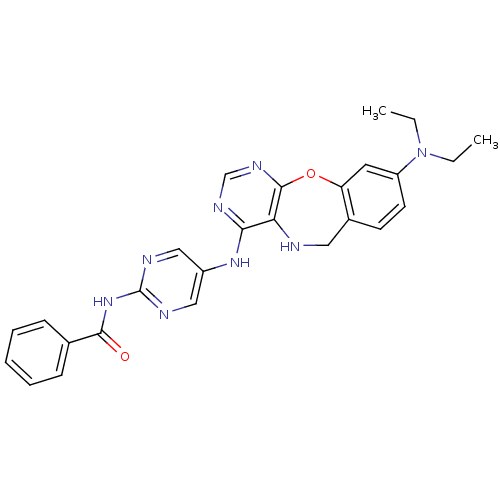
Affinity DataIC50: 470nMAssay Description:Inhibitory activity against Aurora A kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 600nMAssay Description:Inhibitory activity against Aurora A kinaseMore data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein kinase FLT3(Homo sapiens (Human))
Imclone Systems
Curated by ChEMBL
Imclone Systems
Curated by ChEMBL
Affinity DataIC50: 900nMAssay Description:Inhibitory activity against FLT3 kinase in a HTRF assayMore data for this Ligand-Target Pair
Affinity DataIC50: 970nMAssay Description:Inhibitory activity against Aurora A kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+3nMAssay Description:Inhibitory activity against Aurora A kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+3nMAssay Description:Inhibitory activity against Aurora A kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+3nMAssay Description:Inhibitory activity against Aurora A kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.90E+3nMAssay Description:Inhibitory activity against Aurora A kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 2.20E+3nMAssay Description:Inhibitory activity against Aurora A kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 3.50E+3nMAssay Description:Inhibitory activity against Aurora A kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 3.70E+3nMAssay Description:Inhibitory activity against Aurora A kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibitory activity against Aurora A kinaseMore data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 2(Homo sapiens (Human))
Imclone Systems
Curated by ChEMBL
Imclone Systems
Curated by ChEMBL
Affinity DataIC50: 9.50E+3nMAssay Description:Inhibitory activity against KDRMore data for this Ligand-Target Pair
Affinity DataIC50: 9.50E+3nMAssay Description:Inhibitory activity against IRMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibitory activity against Aurora A kinaseMore data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein kinase FLT3(Homo sapiens (Human))
Imclone Systems
Curated by ChEMBL
Imclone Systems
Curated by ChEMBL
Affinity DataIC50: 1.37E+4nMAssay Description:Inhibitory activity against FLT3 kinase in a HTRF assayMore data for this Ligand-Target Pair