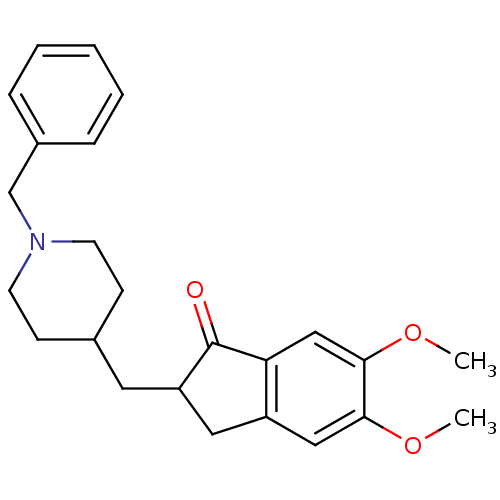
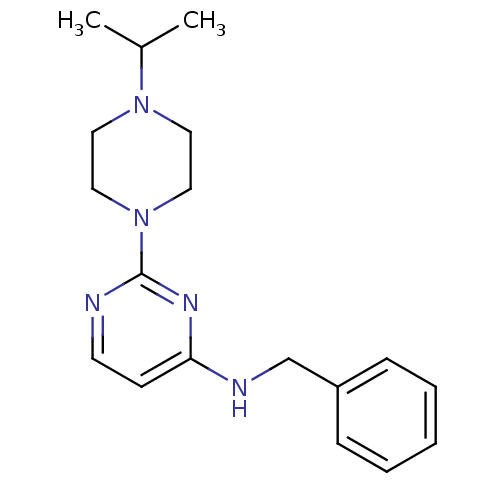
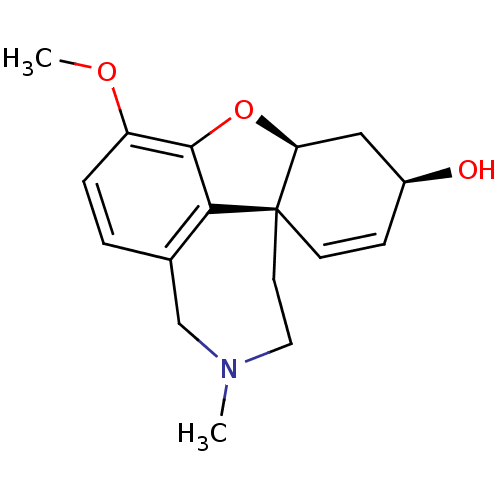
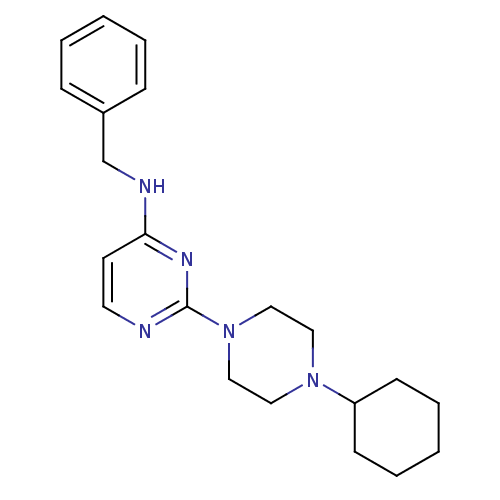
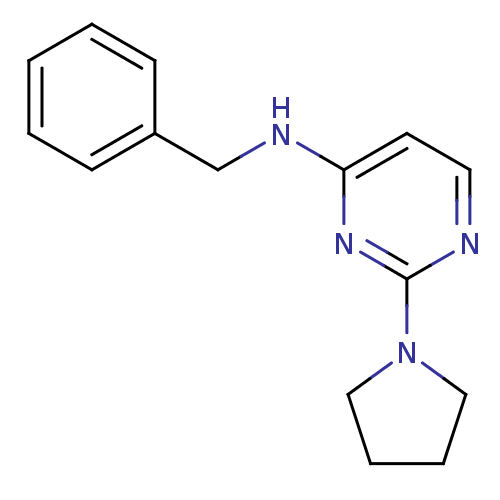
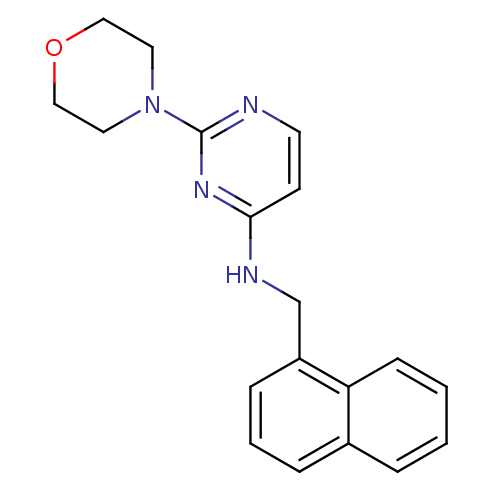
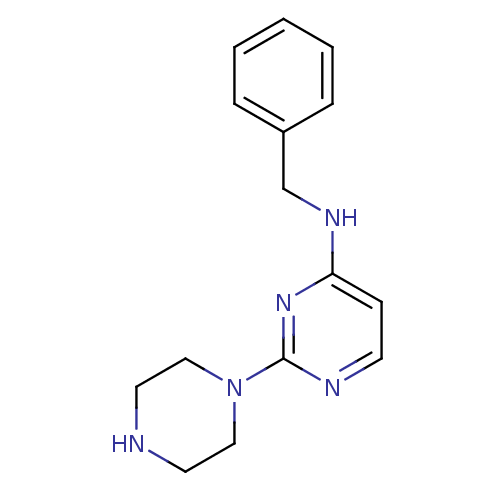
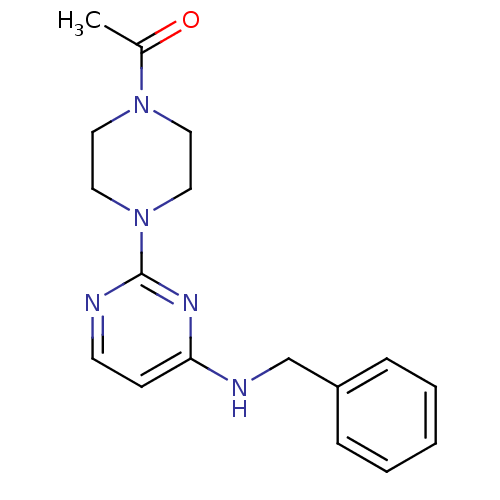
Affinity DataIC50: 4nMAssay Description:Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of equine BChE incubated with compounf for 5 mins before addition of substrate S-butyrylthiocholine iodide by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 19nMAssay Description:Inhibition of equine BChE incubated with compounf for 5 mins before addition of substrate S-butyrylthiocholine iodide by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 32nMAssay Description:Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 93nMAssay Description:Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.20E+3nMAssay Description:Inhibition of equine BChE incubated with compounf for 5 mins before addition of substrate S-butyrylthiocholine iodide by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.60E+3nMAssay Description:Inhibition of equine BChE incubated with compounf for 5 mins before addition of substrate S-butyrylthiocholine iodide by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.40E+3nMAssay Description:Inhibition of equine BChE incubated with compounf for 5 mins before addition of substrate S-butyrylthiocholine iodide by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.40E+3nMAssay Description:Inhibition of equine BChE incubated with compounf for 5 mins before addition of substrate S-butyrylthiocholine iodide by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.60E+3nMAssay Description:Inhibition of equine BChE incubated with compounf for 5 mins before addition of substrate S-butyrylthiocholine iodide by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.80E+3nMAssay Description:Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+3nMAssay Description:Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 6.10E+3nMAssay Description:Inhibition of equine BChE incubated with compounf for 5 mins before addition of substrate S-butyrylthiocholine iodide by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 6.50E+3nMAssay Description:Inhibition of equine BChE incubated with compounf for 5 mins before addition of substrate S-butyrylthiocholine iodide by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 7.30E+3nMAssay Description:Inhibition of equine BChE incubated with compounf for 5 mins before addition of substrate S-butyrylthiocholine iodide by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 7.60E+3nMAssay Description:Inhibition of equine BChE incubated with compounf for 5 mins before addition of substrate S-butyrylthiocholine iodide by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 8.20E+3nMAssay Description:Inhibition of equine BChE incubated with compounf for 5 mins before addition of substrate S-butyrylthiocholine iodide by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 8.70E+3nMAssay Description:Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 8.80E+3nMAssay Description:Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 8.90E+3nMAssay Description:Inhibition of equine BChE incubated with compounf for 5 mins before addition of substrate S-butyrylthiocholine iodide by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 9.80E+3nMAssay Description:Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.07E+4nMAssay Description:Inhibition of equine BChE incubated with compounf for 5 mins before addition of substrate S-butyrylthiocholine iodide by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.24E+4nMAssay Description:Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.26E+4nMAssay Description:Inhibition of equine BChE incubated with compounf for 5 mins before addition of substrate S-butyrylthiocholine iodide by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.26E+4nMAssay Description:Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.28E+4nMAssay Description:Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.38E+4nMAssay Description:Inhibition of equine BChE incubated with compounf for 5 mins before addition of substrate S-butyrylthiocholine iodide by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40E+4nMAssay Description:Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.42E+4nMAssay Description:Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.43E+4nMAssay Description:Inhibition of equine BChE incubated with compounf for 5 mins before addition of substrate S-butyrylthiocholine iodide by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.47E+4nMAssay Description:Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.53E+4nMAssay Description:Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.55E+4nMAssay Description:Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.66E+4nMAssay Description:Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.75E+4nMAssay Description:Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.77E+4nMAssay Description:Inhibition of equine BChE incubated with compounf for 5 mins before addition of substrate S-butyrylthiocholine iodide by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.84E+4nMAssay Description:Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.88E+4nMAssay Description:Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.97E+4nMAssay Description:Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.99E+4nMAssay Description:Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.02E+4nMAssay Description:Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.04E+4nMAssay Description:Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.16E+4nMAssay Description:Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.29E+4nMAssay Description:Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.32E+4nMAssay Description:Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.42E+4nMAssay Description:Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.49E+4nMAssay Description:Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.55E+4nMAssay Description:Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.58E+4nMAssay Description:Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's methodMore data for this Ligand-Target Pair

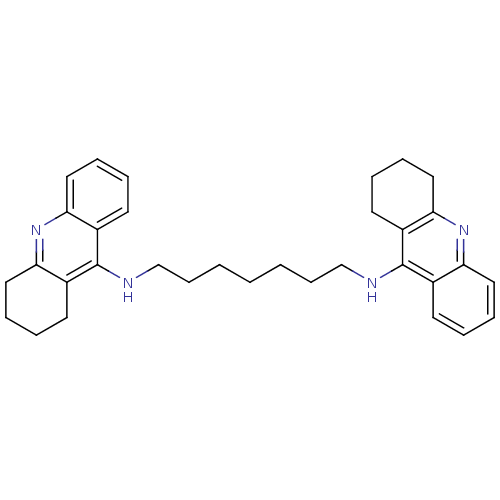
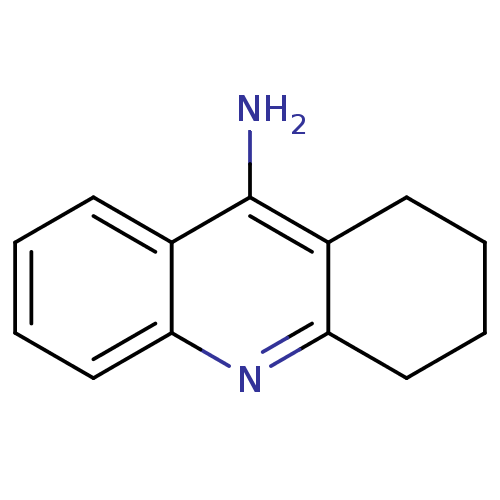
 3D Structure (crystal)
3D Structure (crystal)