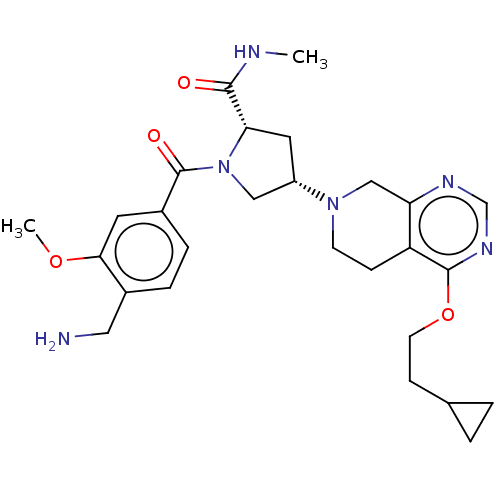
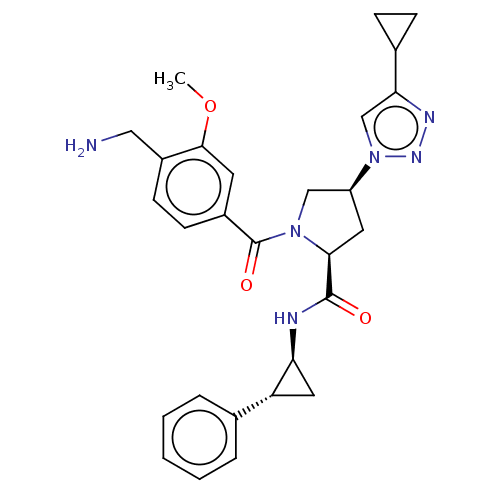
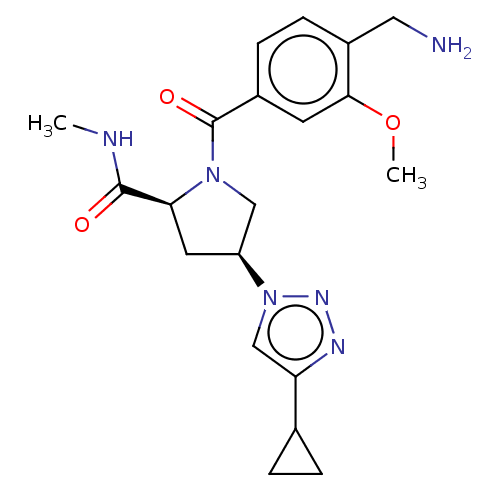
Affinity DataIC50: 2nMAssay Description:Inhibition of human PRSS1 assessed as enzymatic cleavage of Benzoyl-GlyPro-Arg'Rh110-gammaGlu-OH by fluorescence analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of human PRSS1 assessed as enzymatic cleavage of Benzoyl-GlyPro-Arg'Rh110-gammaGlu-OH by fluorescence analysisMore data for this Ligand-Target Pair
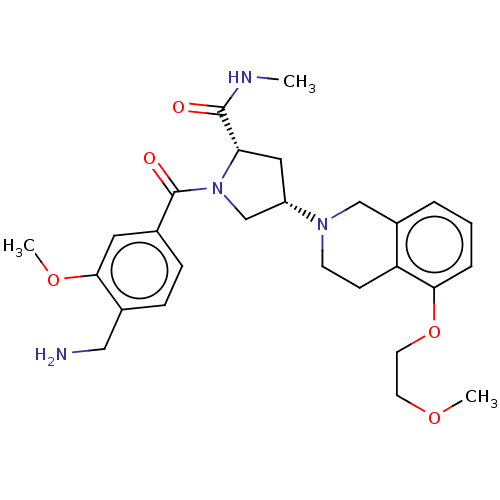
Affinity DataIC50: 3nMAssay Description:Inhibition of human PRSS1 assessed as enzymatic cleavage of Benzoyl-GlyPro-Arg'Rh110-gammaGlu-OH by fluorescence analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of human PRSS1 assessed as enzymatic cleavage of Benzoyl-GlyPro-Arg'Rh110-gammaGlu-OH by fluorescence analysisMore data for this Ligand-Target Pair
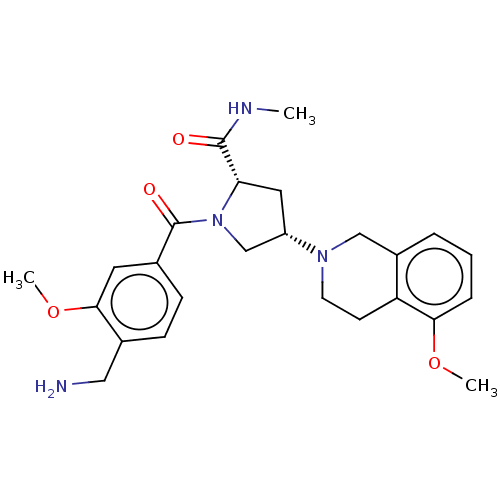
Affinity DataIC50: 5nMAssay Description:Inhibition of human PRSS1 assessed as enzymatic cleavage of Benzoyl-GlyPro-Arg'Rh110-gammaGlu-OH by fluorescence analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of human PRSS1 assessed as enzymatic cleavage of Benzoyl-GlyPro-Arg'Rh110-gammaGlu-OH by fluorescence analysisMore data for this Ligand-Target Pair
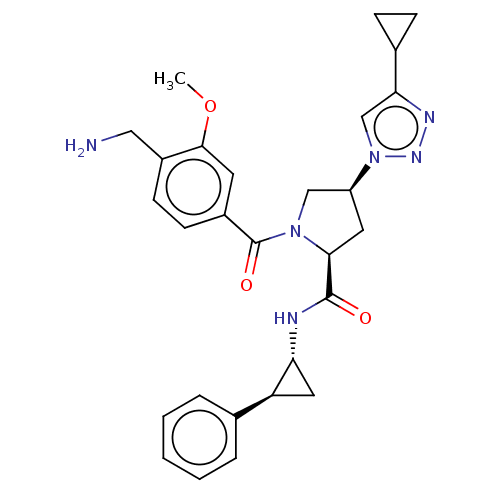
Affinity DataIC50: 6nMAssay Description:Inhibition of human PRSS1 assessed as enzymatic cleavage of Benzoyl-GlyPro-Arg'Rh110-gammaGlu-OH by fluorescence analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of human PRSS1 assessed as enzymatic cleavage of Benzoyl-GlyPro-Arg'Rh110-gammaGlu-OH by fluorescence analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Inhibition of human PRSS1 assessed as enzymatic cleavage of Benzoyl-GlyPro-Arg'Rh110-gammaGlu-OH by fluorescence analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Inhibition of human PRSS1 assessed as enzymatic cleavage of Benzoyl-GlyPro-Arg'Rh110-gammaGlu-OH by fluorescence analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 11nMAssay Description:Inhibition of human PRSS1 assessed as enzymatic cleavage of Benzoyl-GlyPro-Arg'Rh110-gammaGlu-OH by fluorescence analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 11nMAssay Description:Inhibition of human PRSS1 assessed as enzymatic cleavage of Benzoyl-GlyPro-Arg'Rh110-gammaGlu-OH by fluorescence analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 19nMAssay Description:Inhibition of human PRSS1 assessed as enzymatic cleavage of Benzoyl-GlyPro-Arg'Rh110-gammaGlu-OH by fluorescence analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 19nMAssay Description:Inhibition of human PRSS1 assessed as enzymatic cleavage of Benzoyl-GlyPro-Arg'Rh110-gammaGlu-OH by fluorescence analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Inhibition of human PRSS1 assessed as enzymatic cleavage of Benzoyl-GlyPro-Arg'Rh110-gammaGlu-OH by fluorescence analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Inhibition of human PRSS1 assessed as enzymatic cleavage of Benzoyl-GlyPro-Arg'Rh110-gammaGlu-OH by fluorescence analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 110nMAssay Description:Inhibition of human PRSS1 assessed as enzymatic cleavage of Benzoyl-GlyPro-Arg'Rh110-gammaGlu-OH by fluorescence analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 110nMAssay Description:Inhibition of human PRSS1 assessed as enzymatic cleavage of Benzoyl-GlyPro-Arg'Rh110-gammaGlu-OH by fluorescence analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 110nMAssay Description:Inhibition of human PRSS1 assessed as enzymatic cleavage of Benzoyl-GlyPro-Arg'Rh110-gammaGlu-OH by fluorescence analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 110nMAssay Description:Inhibition of human PRSS1 assessed as enzymatic cleavage of Benzoyl-GlyPro-Arg'Rh110-gammaGlu-OH by fluorescence analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 110nMAssay Description:Inhibition of human PRSS1 assessed as enzymatic cleavage of Benzoyl-GlyPro-Arg'Rh110-gammaGlu-OH by fluorescence analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 110nMAssay Description:Inhibition of human PRSS1 assessed as enzymatic cleavage of Benzoyl-GlyPro-Arg'Rh110-gammaGlu-OH by fluorescence analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 160nMAssay Description:Inhibition of human PRSS1 assessed as enzymatic cleavage of Benzoyl-GlyPro-Arg'Rh110-gammaGlu-OH by fluorescence analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 160nMAssay Description:Inhibition of human PRSS1 assessed as enzymatic cleavage of Benzoyl-GlyPro-Arg'Rh110-gammaGlu-OH by fluorescence analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 220nMAssay Description:Inhibition of human PRSS1 assessed as enzymatic cleavage of Benzoyl-GlyPro-Arg'Rh110-gammaGlu-OH by fluorescence analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 220nMAssay Description:Inhibition of human PRSS1 assessed as enzymatic cleavage of Benzoyl-GlyPro-Arg'Rh110-gammaGlu-OH by fluorescence analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 330nMAssay Description:Inhibition of human PRSS1 assessed as enzymatic cleavage of Benzoyl-GlyPro-Arg'Rh110-gammaGlu-OH by fluorescence analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 330nMAssay Description:Inhibition of human PRSS1 assessed as enzymatic cleavage of Benzoyl-GlyPro-Arg'Rh110-gammaGlu-OH by fluorescence analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 490nMAssay Description:Inhibition of human PRSS1 assessed as enzymatic cleavage of Benzoyl-GlyPro-Arg'Rh110-gammaGlu-OH by fluorescence analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 490nMAssay Description:Inhibition of human PRSS1 assessed as enzymatic cleavage of Benzoyl-GlyPro-Arg'Rh110-gammaGlu-OH by fluorescence analysisMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Novartis Pharma
Curated by ChEMBL
Novartis Pharma
Curated by ChEMBL
Affinity DataIC50: 500nMAssay Description:Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cells measured after 90 mins by scintillation proximity assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Novartis Pharma
Curated by ChEMBL
Novartis Pharma
Curated by ChEMBL
Affinity DataIC50: 500nMAssay Description:Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cells measured after 90 mins by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataIC50: 960nMAssay Description:Inhibition of human PRSS1 assessed as enzymatic cleavage of Benzoyl-GlyPro-Arg'Rh110-gammaGlu-OH by fluorescence analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 960nMAssay Description:Inhibition of human PRSS1 assessed as enzymatic cleavage of Benzoyl-GlyPro-Arg'Rh110-gammaGlu-OH by fluorescence analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 2.10E+3nMAssay Description:Inhibition of human PRSS1 assessed as enzymatic cleavage of Benzoyl-GlyPro-Arg'Rh110-gammaGlu-OH by fluorescence analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 2.10E+3nMAssay Description:Inhibition of human PRSS1 assessed as enzymatic cleavage of Benzoyl-GlyPro-Arg'Rh110-gammaGlu-OH by fluorescence analysisMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Novartis Pharma
Curated by ChEMBL
Novartis Pharma
Curated by ChEMBL
Affinity DataIC50: 2.40E+3nMAssay Description:Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cells measured after 90 mins by scintillation proximity assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Novartis Pharma
Curated by ChEMBL
Novartis Pharma
Curated by ChEMBL
Affinity DataIC50: 2.40E+3nMAssay Description:Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cells measured after 90 mins by scintillation proximity assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Novartis Pharma
Curated by ChEMBL
Novartis Pharma
Curated by ChEMBL
Affinity DataIC50: 4.00E+3nMAssay Description:Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cells measured after 90 mins by scintillation proximity assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Novartis Pharma
Curated by ChEMBL
Novartis Pharma
Curated by ChEMBL
Affinity DataIC50: 4.00E+3nMAssay Description:Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cells measured after 90 mins by scintillation proximity assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Novartis Pharma
Curated by ChEMBL
Novartis Pharma
Curated by ChEMBL
Affinity DataIC50: 1.10E+4nMAssay Description:Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cells measured after 90 mins by scintillation proximity assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Novartis Pharma
Curated by ChEMBL
Novartis Pharma
Curated by ChEMBL
Affinity DataIC50: 1.10E+4nMAssay Description:Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cells measured after 90 mins by scintillation proximity assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Novartis Pharma
Curated by ChEMBL
Novartis Pharma
Curated by ChEMBL
Affinity DataIC50: 1.20E+4nMAssay Description:Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cells measured after 90 mins by scintillation proximity assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Novartis Pharma
Curated by ChEMBL
Novartis Pharma
Curated by ChEMBL
Affinity DataIC50: 1.20E+4nMAssay Description:Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cells measured after 90 mins by scintillation proximity assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Novartis Pharma
Curated by ChEMBL
Novartis Pharma
Curated by ChEMBL
Affinity DataIC50: 1.40E+4nMAssay Description:Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cells measured after 90 mins by scintillation proximity assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Novartis Pharma
Curated by ChEMBL
Novartis Pharma
Curated by ChEMBL
Affinity DataIC50: 1.40E+4nMAssay Description:Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cells measured after 90 mins by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+4nMAssay Description:Inhibition of human chymotrypsinMore data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+4nMAssay Description:Inhibition of human chymotrypsinMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Novartis Pharma
Curated by ChEMBL
Novartis Pharma
Curated by ChEMBL
Affinity DataIC50: 2.20E+4nMAssay Description:Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cells measured after 90 mins by scintillation proximity assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Novartis Pharma
Curated by ChEMBL
Novartis Pharma
Curated by ChEMBL
Affinity DataIC50: 2.20E+4nMAssay Description:Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cells measured after 90 mins by scintillation proximity assayMore data for this Ligand-Target Pair