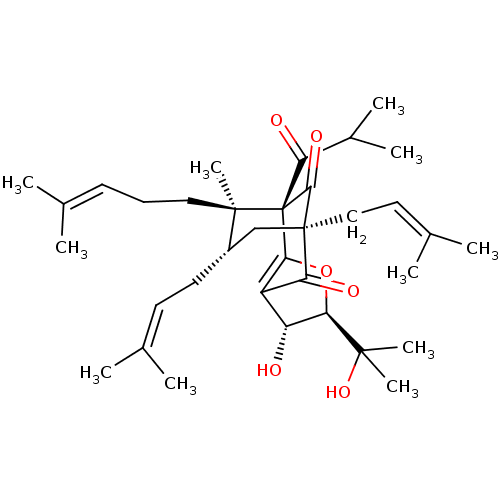
Affinity DataIC50: 270nMAssay Description:Inhibition of human erythrocyte AChE using S-acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured e...More data for this Ligand-Target Pair
Affinity DataIC50: 3.98E+3nMAssay Description:Inhibition of human erythrocyte AChE using S-acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured e...More data for this Ligand-Target Pair
Affinity DataIC50: 7.17E+3nMAssay Description:Inhibition of human erythrocyte AChE using S-acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured e...More data for this Ligand-Target Pair
Affinity DataIC50: 8.75E+3nMAssay Description:Inhibition of human erythrocyte AChE using S-acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured e...More data for this Ligand-Target Pair
Affinity DataIC50: 8.83E+3nMAssay Description:Inhibition of human erythrocyte AChE using S-acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured e...More data for this Ligand-Target Pair
Affinity DataIC50: 9.13E+3nMAssay Description:Inhibition of human erythrocyte AChE using S-acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured e...More data for this Ligand-Target Pair
Affinity DataIC50: 1.07E+4nMAssay Description:Inhibition of human erythrocyte AChE using S-acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured e...More data for this Ligand-Target Pair
Affinity DataIC50: 1.09E+4nMAssay Description:Inhibition of human erythrocyte AChE using S-acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured e...More data for this Ligand-Target Pair
Affinity DataIC50: 1.13E+4nMAssay Description:Inhibition of human erythrocyte AChE using S-acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured e...More data for this Ligand-Target Pair
Affinity DataIC50: 1.26E+4nMAssay Description:Inhibition of human erythrocyte AChE using S-acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured e...More data for this Ligand-Target Pair
Affinity DataIC50: 1.86E+4nMAssay Description:Inhibition of human erythrocyte AChE using S-acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured e...More data for this Ligand-Target Pair
Affinity DataIC50: 1.96E+4nMAssay Description:Inhibition of human erythrocyte AChE using S-acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured e...More data for this Ligand-Target Pair
Affinity DataIC50: 2.43E+4nMAssay Description:Inhibition of human erythrocyte AChE using S-acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured e...More data for this Ligand-Target Pair
Affinity DataIC50: 2.51E+4nMAssay Description:Inhibition of human erythrocyte AChE using S-acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured e...More data for this Ligand-Target Pair
Affinity DataIC50: 2.64E+4nMAssay Description:Inhibition of human erythrocyte AChE using S-acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured e...More data for this Ligand-Target Pair

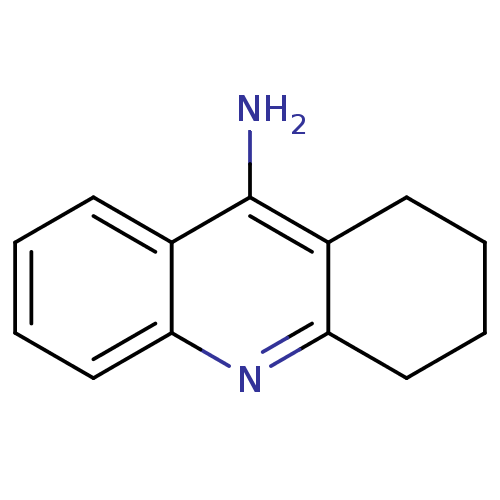
 3D Structure (crystal)
3D Structure (crystal)