Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Melanocortin receptor 4
Ligand
BDBM50491635
Substrate
n/a
Meas. Tech.
ChEMBL_961358 (CHEMBL2390685)
IC50
12±n/a nM
Citation
 Brabez, N; Nguyen, KL; Saunders, K; Lacy, R; Xu, L; Gillies, RJ; Lynch, RM; Chassaing, G; Lavielle, S; Hruby, VJ Synthesis and evaluation of cholecystokinin trimers: a multivalent approach to pancreatic cancer detection and treatment. Bioorg Med Chem Lett 23:2422-5 (2013) [PubMed] Article
Brabez, N; Nguyen, KL; Saunders, K; Lacy, R; Xu, L; Gillies, RJ; Lynch, RM; Chassaing, G; Lavielle, S; Hruby, VJ Synthesis and evaluation of cholecystokinin trimers: a multivalent approach to pancreatic cancer detection and treatment. Bioorg Med Chem Lett 23:2422-5 (2013) [PubMed] Article More Info.:
Target
Name:
Melanocortin receptor 4
Synonyms:
MC4-R | MC4R | MC4R_HUMAN | Melanocortin MC4 | Melanocortin receptor 4 (MC-4) | Melanocortin receptor 4 (MC4-R) | Melanocortin receptor 4 (MC4R)
Type:
Enzyme
Mol. Mass.:
36949.50
Organism:
Homo sapiens (Human)
Description:
P32245
Residue:
332
Sequence:
MVNSTHRGMHTSLHLWNRSSYRLHSNASESLGKGYSDGGCYEQLFVSPEVFVTLGVISLLENILVIVAIAKNKNLHSPMYFFICSLAVADMLVSVSNGSETIVITLLNSTDTDAQSFTVNIDNVIDSVICSSLLASICSLLSIAVDRYFTIFYALQYHNIMTVKRVGIIISCIWAACTVSGILFIIYSDSSAVIICLITMFFTMLALMASLYVHMFLMARLHIKRIAVLPGTGAIRQGANMKGAITLTILIGVFVVCWAPFFLHLIFYISCPQNPYCVCFMSHFNLYLILIMCNSIIDPLIYALRSQELRKTFKEIICCYPLGGLCDLSSRY
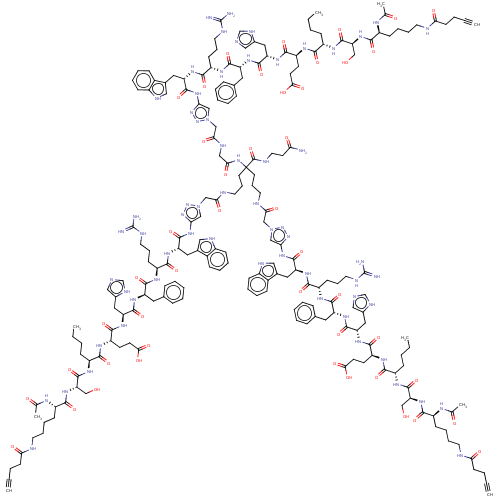
Inhibitor
Name:
BDBM50491635
Synonyms:
CHEMBL2386882
Type:
Small organic molecule
Emp. Form.:
C202H277N63O45
Mol. Mass.:
4307.7559
SMILES:
CCCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](CCCCNC(=O)CCC#C)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)Nc1cn(CC(=O)NCCCC(CCCNC(=O)Cn2cc(NC(=O)[C@H](Cc3c[nH]c4ccccc34)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc3ccccc3)NC(=O)[C@H](Cc3cnc[nH]3)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCC)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCNC(=O)CCC#C)NC(C)=O)nn2)(NC(=O)CNC(=O)Cn2cc(NC(=O)[C@H](Cc3c[nH]c4ccccc34)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc3ccccc3)NC(=O)[C@H](Cc3cnc[nH]3)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCC)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCNC(=O)CCC#C)NC(C)=O)nn2)C(=O)NCCC(N)=O)nn1 |r|