Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Botulinum neurotoxin type A
Ligand
BDBM50111498
Substrate
n/a
Meas. Tech.
ChEMBL_1510137 (CHEMBL3606427)
IC50
157000±n/a nM
Citation
 Teng, YH; Berger, WT; Nesbitt, NM; Kumar, K; Balius, TE; Rizzo, RC; Tonge, PJ; Ojima, I; Swaminathan, S Computer-aided identification, synthesis, and biological evaluation of novel inhibitors for botulinum neurotoxin serotype A. Bioorg Med Chem 23:5489-95 (2015) [PubMed] Article
Teng, YH; Berger, WT; Nesbitt, NM; Kumar, K; Balius, TE; Rizzo, RC; Tonge, PJ; Ojima, I; Swaminathan, S Computer-aided identification, synthesis, and biological evaluation of novel inhibitors for botulinum neurotoxin serotype A. Bioorg Med Chem 23:5489-95 (2015) [PubMed] Article More Info.:
Target
Name:
Botulinum neurotoxin type A
Synonyms:
BXA1_CLOBO | atx | bonT | botA
Type:
PROTEIN
Mol. Mass.:
149450.01
Organism:
Clostridium botulinum
Description:
ChEMBL_1510137
Residue:
1296
Sequence:
MPFVNKQFNYKDPVNGVDIAYIKIPNVGQMQPVKAFKIHNKIWVIPERDTFTNPEEGDLNPPPEAKQVPVSYYDSTYLSTDNEKDNYLKGVTKLFERIYSTDLGRMLLTSIVRGIPFWGGSTIDTELKVIDTNCINVIQPDGSYRSEELNLVIIGPSADIIQFECKSFGHEVLNLTRNGYGSTQYIRFSPDFTFGFEESLEVDTNPLLGAGKFATDPAVTLAHELIHAGHRLYGIAINPNRVFKVNTNAYYEMSGLEVSFEELRTFGGHDAKFIDSLQENEFRLYYYNKFKDIASTLNKAKSIVGTTASLQYMKNVFKEKYLLSEDTSGKFSVDKLKFDKLYKMLTEIYTEDNFVKFFKVLNRKTYLNFDKAVFKINIVPKVNYTIYDGFNLRNTNLAANFNGQNTEINNMNFTKLKNFTGLFEFYKLLCVRGIITSKTKSLDKGYNKALNDLCIKVNNWDLFFSPSEDNFTNDLNKGEEITSDTNIEAAEENISLDLIQQYYLTFNFDNEPENISIENLSSDIIGQLELMPNIERFPNGKKYELDKYTMFHYLRAQEFEHGKSRIALTNSVNEALLNPSRVYTFFSSDYVKKVNKATEAAMFLGWVEQLVYDFTDETSEVSTTDKIADITIIIPYIGPALNIGNMLYKDDFVGALIFSGAVILLEFIPEIAIPVLGTFALVSYIANKVLTVQTIDNALSKRNEKWDEVYKYIVTNWLAKVNTQIDLIRKKMKEALENQAEATKAIINYQYNQYTEEEKNNINFNIDDLSSKLNESINKAMININKFLNQCSVSYLMNSMIPYGVKRLEDFDASLKDALLKYIYDNRGTLIGQVDRLKDKVNNTLSTDIPFQLSKYVDNQRLLSTFTEYIKNIINTSILNLRYESNHLIDLSRYASKINIGSKVNFDPIDKNQIQLFNLESSKIEVILKNAIVYNSMYENFSTSFWIRIPKYFNSISLNNEYTIINCMENNSGWKVSLNYGEIIWTLQDTQEIKQRVVFKYSQMINISDYINRWIFVTITNNRLNNSKIYINGRLIDQKPISNLGNIHASNNIMFKLDGCRDTHRYIWIKYFNLFDKELNEKEIKDLYDNQSNSGILKDFWGDYLQYDKPYYMLNLYDPNKYVDVNNVGIRGYMYLKGPRGSVMTTNIYLNSSLYRGTKFIIKKYASGNKDNIVRNNDRVYINVVVKNKEYRLATNASQAGVEKILSALEIPDVGNLSQVVVMKSKNDQGITNKCKMNLQDNNGNDIGFIGFHQFNNIAKLVASNWYNRQIERSSRTLGCSWEFIPVDDGWGERPL
Inhibitor
Name:
BDBM50111498
Synonyms:
CHEMBL3604950
Type:
Small organic molecule
Emp. Form.:
C24H28N2O3
Mol. Mass.:
392.4907
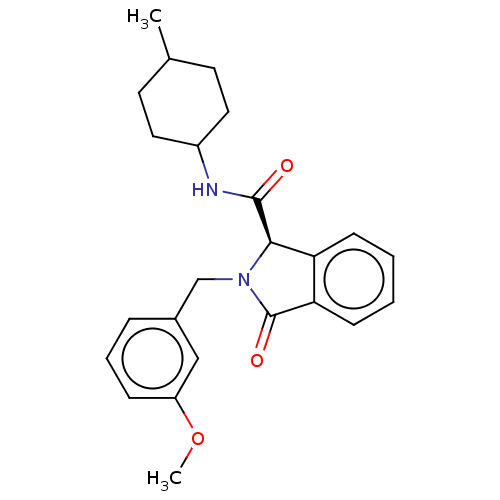
SMILES:
COc1cccc(CN2[C@@H](C(=O)NC3CCC(C)CC3)c3ccccc3C2=O)c1 |r,wD:9.9,(3.03,-5.31,;4.26,-5.29,;5.02,-3.95,;6.56,-3.93,;7.31,-2.59,;6.53,-1.27,;4.99,-1.28,;4.2,.04,;2.66,.02,;1.76,1.24,;2.24,2.7,;3.45,2.95,;1.21,3.85,;1.69,5.31,;.67,6.46,;1.15,7.92,;2.66,8.23,;3.05,9.4,;3.68,7.08,;3.2,5.62,;.3,.77,;-1.03,1.55,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.15,-2.41,;4.23,-2.63,)|