Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Bromodomain-containing protein 4 [1-477]
Ligand
BDBM445554
Substrate
n/a
Meas. Tech.
Time Resolved Fluorescence Resonance Energy Transfer (TR-FRET) Assay
IC50
<5±n/a nM
Citation
 Quesnelle, CA; Harikrishnan, LS; Hill, MD Tricyclic compounds as anticancer agents US Patent US10683290 Publication Date 6/16/2020
Quesnelle, CA; Harikrishnan, LS; Hill, MD Tricyclic compounds as anticancer agents US Patent US10683290 Publication Date 6/16/2020 More Info.:
Target
Name:
Bromodomain-containing protein 4 [1-477]
Synonyms:
BRD4 | BRD4_HUMAN | Bromodomain-containing protein 4 (BRD4)(1-477) | HUNK1
Type:
Enzyme Catalytic Domain
Mol. Mass.:
52863.68
Organism:
Homo sapiens (Human)
Description:
O60885[1-477]
Residue:
475
Sequence:
MSAESGPGTRLRNLPVMGDGLETSQMSTTQAQAQPQPANAASTNPPPPETSNPNKPKRQTNQLQYLLRVVLKTLWKHQFAWPFQQPVDAVKLNLPDYYKIIKTPMDMGTIKKRLENNYYWNAQECIQDFNTMFTNCYIYNKPGDDIVLMAEALEKLFLQKINELPTEETEIMIVQAKGRGRGRKETGTAKPGVSTVPNTTQASTPPQTQTPQPNPPPVQATPHPFPAVTPDLIVQTPVMTVVPPQPLQTPPPVPPQPQPPPAPAPQPVQSHPPIIAATPQPVKTKKGVKRKADTTTPTTIDPIHEPPSLPPEPKTTKLGQRRESSRPVKPPKKDVPDSQQHPAPEKSSKVSEQLKCCSGILKEMFAKKHAAYAWPFYKPVDVEALGLHDYCDIIKHPMDMSTIKSKLEAREYRDAQEFGADVRLMFSNCYKYNPPDHEVVAMARKLQDVFEMRFAKMPDEPEEPVVAVSSPAVPP
Inhibitor
Name:
BDBM445554
Synonyms:
US10683290, Example 52
Type:
Small organic molecule
Emp. Form.:
C30H33FN6O2
Mol. Mass.:
528.6204
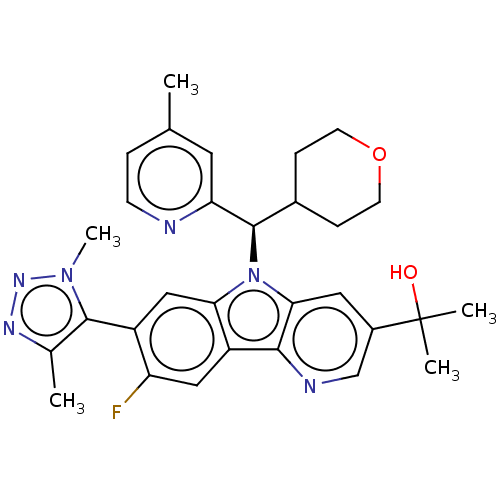
SMILES:
Cc1nnn(C)c1-c1cc2n([C@H](C3CCOCC3)c3cc(C)ccn3)c3cc(cnc3c2cc1F)C(C)(C)O |r,wU:11.11,(4.65,-.42,;5.42,.91,;6.95,1.07,;7.27,2.58,;5.94,3.35,;6.34,4.84,;4.79,2.32,;3.29,2.64,;2.26,1.5,;.75,1.82,;-.5,.91,;-.5,-.63,;-1.83,-1.4,;-3.16,-.63,;-4.5,-1.4,;-4.5,-2.94,;-3.16,-3.71,;-1.83,-2.94,;.84,-1.4,;.84,-2.94,;2.17,-3.71,;2.17,-5.25,;3.51,-2.94,;3.51,-1.4,;2.17,-.63,;-1.74,1.82,;-3.25,1.5,;-4.28,2.64,;-3.8,4.1,;-2.3,4.42,;-1.27,3.28,;.27,3.28,;1.31,4.42,;2.81,4.1,;3.84,5.25,;-5.78,2.32,;-6.81,3.46,;-7.27,1.92,;-6.26,.86,)|