Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Urokinase-type plasminogen activator
Ligand
BDBM14350
Substrate
BDBM13949
Meas. Tech.
Enzyme Assay and Determination of the Inhibition Constants
Ki
33±n/a nM
Citation
 Katz, BA; Elrod, K; Verner, E; Mackman, RL; Luong, C; Shrader, WD; Sendzik, M; Spencer, JR; Sprengeler, PA; Kolesnikov, A; Tai, VW; Hui, HC; Breitenbucher, JG; Allen, D; Janc, JW Elaborate manifold of short hydrogen bond arrays mediating binding of active site-directed serine protease inhibitors. J Mol Biol 329:93-120 (2003) [PubMed] Article
Katz, BA; Elrod, K; Verner, E; Mackman, RL; Luong, C; Shrader, WD; Sendzik, M; Spencer, JR; Sprengeler, PA; Kolesnikov, A; Tai, VW; Hui, HC; Breitenbucher, JG; Allen, D; Janc, JW Elaborate manifold of short hydrogen bond arrays mediating binding of active site-directed serine protease inhibitors. J Mol Biol 329:93-120 (2003) [PubMed] Article More Info.:
Target
Name:
Urokinase-type plasminogen activator
Synonyms:
3.4.21.73 | PLAU | U-plasminogen activator | UROK_HUMAN | Urokinase | Urokinase-type plasminogen activator (uPA) | Urokinase-type plasminogen activator chain B | Urokinase-type plasminogen activator long chain A | Urokinase-type plasminogen activator short chain A | Urokinase-type plasminogen activator/surface receptor | uPA
Type:
Enzyme
Mol. Mass.:
48528.62
Organism:
Homo sapiens (Human)
Description:
P00749
Residue:
431
Sequence:
MRALLARLLLCVLVVSDSKGSNELHQVPSNCDCLNGGTCVSNKYFSNIHWCNCPKKFGGQHCEIDKSKTCYEGNGHFYRGKASTDTMGRPCLPWNSATVLQQTYHAHRSDALQLGLGKHNYCRNPDNRRRPWCYVQVGLKLLVQECMVHDCADGKKPSSPPEELKFQCGQKTLRPRFKIIGGEFTTIENQPWFAAIYRRHRGGSVTYVCGGSLISPCWVISATHCFIDYPKKEDYIVYLGRSRLNSNTQGEMKFEVENLILHKDYSADTLAHHNDIALLKIRSKEGRCAQPSRTIQTICLPSMYNDPQFGTSCEITGFGKENSTDYLYPEQLKMTVVKLISHRECQQPHYYGSEVTTKMLCAADPQWKTDSCQGDSGGPLVCSLQGRMTLTGIVSWGRGCALKDKPGVYTRVSHFLPWIRSHTKEENGLAL
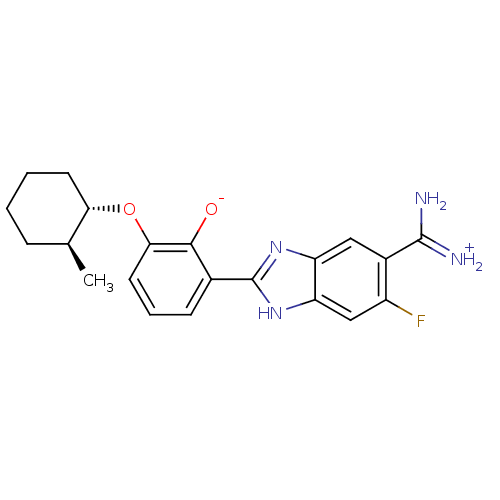
Inhibitor
Name:
BDBM14350
Synonyms:
2-{5-[amino(iminiumyl)methyl]-6-fluoro-1H-1,3-benzodiazol-2-yl}-6-{[(1S,2S)-2-methylcyclohexyl]oxy}benzen-1-olate | CA-11 | CRA-11092
Type:
Small organic molecule
Emp. Form.:
C21H23FN4O2
Mol. Mass.:
382.4313
SMILES:
C[C@H]1CCCC[C@@H]1Oc1cccc(-c2nc3cc(C(N)=[NH2+])c(F)cc3[nH]2)c1[O-] |r|
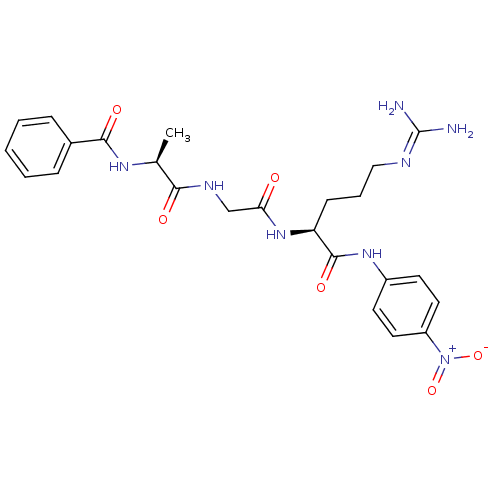
Substrate
Name:
BDBM13949
Synonyms:
(2S)-5-carbamimidamido-N-(4-nitrophenyl)-2-{2-[(2S)-2-(phenylformamido)propanamido]acetamido}pentanamide | Bz-Ala-Gly-Arg-pNA | uPA Chromogenic Substrate
Type:
Small organic molecule
Emp. Form.:
C24H30N8O6
Mol. Mass.:
526.545
SMILES:
[#6]-[#6@H](-[#7]-[#6](=O)-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-c1ccc(cc1)-[#7+](-[#8-])=O |r|