Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Genome polyprotein
Ligand
BDBM357840
Substrate
n/a
Meas. Tech.
HCV Replicon Luciferase Assay
EC50
6.10±n/a nM
Citation
 Wang, T; Zhang, Z; Parcella, KE; Eastman, KJ; Kadow, JF Substituted 2-phenyl (AZA)benzofuran compounds for the treatment of hepatitis C US Patent US10214534 Publication Date 2/26/2019
Wang, T; Zhang, Z; Parcella, KE; Eastman, KJ; Kadow, JF Substituted 2-phenyl (AZA)benzofuran compounds for the treatment of hepatitis C US Patent US10214534 Publication Date 2/26/2019 More Info.:
Target
Name:
Genome polyprotein
Synonyms:
HCV Polymerase (S282T) | NS3 serine protease (NS3) | NS3/4A Protein | NS3/4a Protease | POLG_HCVJA
Type:
Protein
Mol. Mass.:
327076.78
Organism:
Hepatitis C Virus (Virus)
Description:
P26662
Residue:
3010
Sequence:
MSTNPKPQRKTKRNTNRRPQDVKFPGGGQIVGGVYLLPRRGPRLGVRATRKTSERSQPRGRRQPIPKARRPEGRTWAQPGYPWPLYGNEGMGWAGWLLSPRGSRPSWGPTDPRRRSRNLGKVIDTLTCGFADLMGYIPLVGAPLGGAARALAHGVRVLEDGVNYATGNLPGCSFSIFLLALLSCLTIPASAYEVRNVSGIYHVTNDCSNSSIVYEAADMIMHTPGCVPCVRESNFSRCWVALTPTLAARNSSIPTTTIRRHVDLLVGAAALCSAMYVGDLCGSVFLVSQLFTFSPRRYETVQDCNCSIYPGHVSGHRMAWDMMMNWSPTTALVVSQLLRIPQAVVDMVAGAHWGVLAGLAYYSMVGNWAKVLIVMLLFAGVDGHTHVTGGRVASSTQSLVSWLSQGPSQKIQLVNTNGSWHINRTALNCNDSLQTGFIAALFYAHRFNASGCPERMASCRPIDEFAQGWGPITHDMPESSDQRPYCWHYAPRPCGIVPASQVCGPVYCFTPSPVVVGTTDRFGAPTYSWGENETDVLLLSNTRPPQGNWFGCTWMNSTGFTKTCGGPPCNIGGVGNNTLVCPTDCFRKHPEATYTKCGSGPWLTPRCMVDYPYRLWHYPCTVNFTVFKVRMYVGGVEHRLNAACNWTRGERCDLEDRDRSELSPLLLSTTEWQILPCSFTTLPALSTGLIHLHRNIVDVQYLYGIGSAVVSFAIKWEYILLLFLLLADARVCACLWMMLLIAQAEATLENLVVLNAASVAGAHGLLSFLVFFCAAWYIKGRLVPGAAYALYGVWPLLLLLLALPPRAYAMDREMAASCGGAVFVGLVLLTLSPYYKVFLARLIWWLQYFITRAEAHLQVWVPPLNVRGGRDAIILLTCAVHPELIFDITKLLLAILGPLMVLQAGITRVPYFVRAQGLIRACMLVRKVAGGHYVQMAFMKLAALTGTYVYDHLTPLRDWAHAGLRDLAVAVEPVVFSDMETKLITWGADTAACGDIISGLPVSARRGKEILLGPADSFGEQGWRLLAPITAYSQQTRGLLGCIITSLTGRDKNQVDGEVQVLSTATQSFLATCVNGVCWTVYHGAGSKTLAGPKGPITQMYTNVDQDLVGWPAPPGARSMTPCTCGSSDLYLVTRHADVVPVRRRGDSRGSLLSPRPISYLKGSSGGPLLCPSGHVVGIFRAAVCTRGVAKAVDFIPVESMETTMRSPVFTDNSSPPAVPQTFQVAHLHAPTGSGKSTKVPAAYAAQGYKVLVLNPSVAATLGFGAYMSKAHGIEPNIRTGVRTITTGGPITYSTYCKFLADGGCSGGAYDIIICDECHSTDSTTILGIGTVLDQAETAGARLVVLATATPPGSITVPHPNIEEVALSNTGEIPFYGKAIPIEAIKGGRHLIFCHSKKKCDELAAKLTGLGLNAVAYYRGLDVSVIPTSGDVVVVATDALMTGFTGDFDSVIDCNTCVTQTVDFSLDPTFTIETTTLPQDAVSRAQRRGRTGRGRSGIYRFVTPGERPSGMFDSSVLCECYDAGCAWYELTPAETSVRLRAYLNTPGLPVCQDHLEFWESVFTGLTHIDAHFLSQTKQAGDNLPYLVAYQATVCARAQAPPPSWDQMWKCLIRLKPTLHGPTPLLYRLGAVQNEVTLTHPITKYIMACMSADLEVVTSTWVLVGGVLAALAAYCLTTGSVVIVGRIILSGRPAVIPDREVLYQEFDEMEECASHLPYIEQGMQLAEQFKQKALGLLQTATKQAEAAAPVVESKWRALEVFWAKHMWNFISGIQYLAGLSTLPGNPAIASLMAFTASITSPLTTQNTLLFNILGGWVAAQLAPPSAASAFVGAGIAGAAVGSIGLGKVLVDILAGYGAGVAGALVAFKVMSGEMPSTEDLVNLLPAILSPGALVVGVVCAAILRRHVGPGEGAVQWMNRLIAFASRGNHVSPTHYVPESDAAARVTQILSSLTITQLLKRLHQWINEDCSTPCSGSWLKDVWDWICTVLSDFKTWLQSKLLPRLPGLPFLSCQRGYKGVWRGDGIMQTTCPCGAQITGHVKNGSMRIVGPKTCSNTWHGTFPINAYTTGPCTPSPAPNYSRALWRVAAEEYVEVTRVGDFHYVTGMTTDNVKCPCQVPAPEFFTEVDGVRLHRYAPVCKPLLREEVVFQVGLNQYLVGSQLPCEPEPDVAVLTSMLTDPSHITAETAKRRLARGSPPSLASSSASQLSAPSLKATCTTHHDSPDADLIEANLLWRQEMGGNITRVESENKVVILDSFDPIRAVEDEREISVPAEILRKPRKFPPALPIWARPDYNPPLLESWKDPDYVPPVVHGCPLPSTKAPPIPPPRRKRTVVLTESTVSSALAELATKTFGSSGSSAVDSGTATGPPDQASDDGDKGSDVESYSSMPPLEGEPGDPDLSDGSWSTVSGEAGEDVVCCSMSYTWTGALITPCAAEESKLPINPLSNSLLRHHSMVYSTTSRSASLRQKKVTFDRLQVLDDHYRDVLKEMKAKASTVKARLLSIEEACKLTPPHSAKSKFGYGAKDVRSLSSRAVNHIRSVWEDLLEDTETPIDTTIMAKNEVFCVQPEKGGRKPARLIVFPDLGVRVCEKMALYDVVSTLPQAVMGPSYGFQYSPGQRVEFLVNTWKSKKCPMGFSYDTRCFDSTVTENDIRTEESIYQCCDLAPEARQAIRSLTERLYVGGPLTNSKGQNCGYRRCRASGVLTTSCGNTLTCYLKATAACRAAKLQDCTMLVNGDDLVVICESAGTQEDAAALRAFTEAMTRYSAPPGDPPQPEYDLELITSCSSNVSVAHDASGKRVYYLTRDPTTPLARAAWETVRHTPVNSWLGNIIMYAPTLWARMILMTHFFSILLAQEQLEKALDCQIYGACYSIEPLDLPQIIERLHGLSAFSLHSYSPGEINRVASCLRKLGVPPLRVWRHRARSVRAKLLSQGGRAATCGKYLFNWAVKTKLKLTPIPAASQLDLSGWFVAGYNGGDIYHSLSRARPRWFMLCLLLLSVGVGIYLLPNR
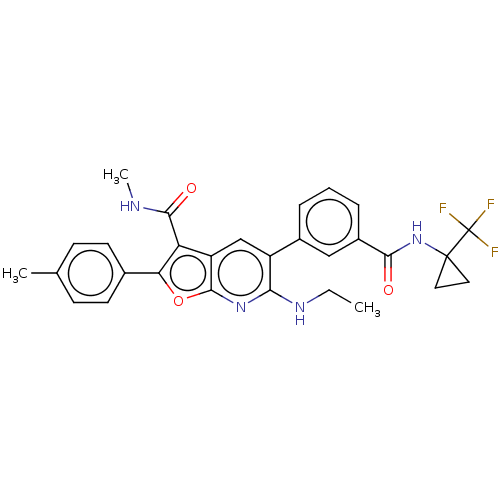
Inhibitor
Name:
BDBM357840
Synonyms:
Step 1: Preparation of 6-chloro-N-methyl-2-(p-tolyl)-5-(3-((1-(trifluoromethyl)cyclopropyl)carbamoyl)phenyl)furo[2,3-b]pyridine-3-carboxamide | US10214534, Compound 6003
Type:
Small organic molecule
Emp. Form.:
C29H27F3N4O3
Mol. Mass.:
536.5449
SMILES:
CCNc1nc2oc(c(C(=O)NC)c2cc1-c1cccc(c1)C(=O)NC1(CC1)C(F)(F)F)-c1ccc(C)cc1