Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Bifunctional purine biosynthesis protein ATIC
Ligand
BDBM22587
Substrate
BDBM22579
Meas. Tech.
AICAR Tfase Inhibition Assay
pH
7.5±n/a
Temperature
295.15±n/a K
Ki
>100000±n/a nM
Citation
 Xu, L; Chong, Y; Hwang, I; D'Onofrio, A; Amore, K; Beardsley, GP; Li, C; Olson, AJ; Boger, DL; Wilson, IA Structure-based design, synthesis, evaluation, and crystal structures of transition state analogue inhibitors of inosine monophosphate cyclohydrolase. J Biol Chem 282:13033-46 (2007) [PubMed] Article
Xu, L; Chong, Y; Hwang, I; D'Onofrio, A; Amore, K; Beardsley, GP; Li, C; Olson, AJ; Boger, DL; Wilson, IA Structure-based design, synthesis, evaluation, and crystal structures of transition state analogue inhibitors of inosine monophosphate cyclohydrolase. J Biol Chem 282:13033-46 (2007) [PubMed] Article More Info.:
Target
Name:
Bifunctional purine biosynthesis protein ATIC
Synonyms:
5-aminoimidazole-4-carboxamide ribonucleotide formyltransferase | 5-aminoimidazole-4-carboxamide-ribonucleotide transformylase | AICAR Tfase | AICAR transformylase | ATIC | Aminoimidazole carboxamide ribonucleotide transformylase (AICAR Tfase) | Bifunctional purine biosynthesis protein PURH | IMP Cyclohydrolase (IMPCH) | IMP cyclohydrolase | IMP synthetase | Inosinicase | PUR9_HUMAN | PURH | Phosphoribosylaminoimidazolecarboxamide formyltransferase | Thymidylate synthase/GAR transformylase/AICAR transformylase
Type:
Protein
Mol. Mass.:
64616.62
Organism:
Homo sapiens (Human)
Description:
P31939
Residue:
592
Sequence:
MAPGQLALFSVSDKTGLVEFARNLTALGLNLVASGGTAKALRDAGLAVRDVSELTGFPEMLGGRVKTLHPAVHAGILARNIPEDNADMARLDFNLIRVVACNLYPFVKTVASPGVTVEEAVEQIDIGGVTLLRAAAKNHARVTVVCEPEDYVVVSTEMQSSESKDTSLETRRQLALKAFTHTAQYDEAISDYFRKQYSKGVSQMPLRYGMNPHQTPAQLYTLQPKLPITVLNGAPGFINLCDALNAWQLVKELKEALGIPAAASFKHVSPAGAAVGIPLSEDEAKVCMVYDLYKTLTPISAAYARARGADRMSSFGDFVALSDVCDVPTAKIISREVSDGIIAPGYEEEALTILSKKKNGNYCVLQMDQSYKPDENEVRTLFGLHLSQKRNNGVVDKSLFSNVVTKNKDLPESALRDLIVATIAVKYTQSNSVCYAKNGQVIGIGAGQQSRIHCTRLAGDKANYWWLRHHPQVLSMKFKTGVKRAEISNAIDQYVTGTIGEDEDLIKWKALFEEVPELLTEAEKKEWVEKLTEVSISSDAFFPFRDNVDRAKRSGVAYIAAPSGSAADKVVIEACDELGIILAHTNLRLFHH
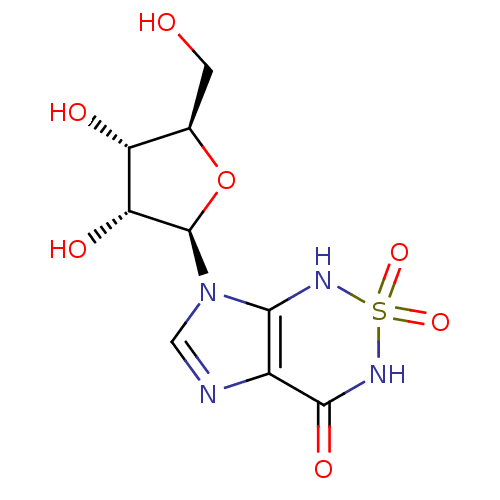
Inhibitor
Name:
BDBM22587
Synonyms:
7-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-1H,3H,4H,7H-2,1,3,5,7-imidazo[4,5-c][1,2,6]thiadiazine-2,2,4-trione | Nucleoside, 2
Type:
Nucleoside or nucleotide
Emp. Form.:
C9H12N4O7S
Mol. Mass.:
320.279
SMILES:
OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c1NS(=O)(=O)NC2=O |r|
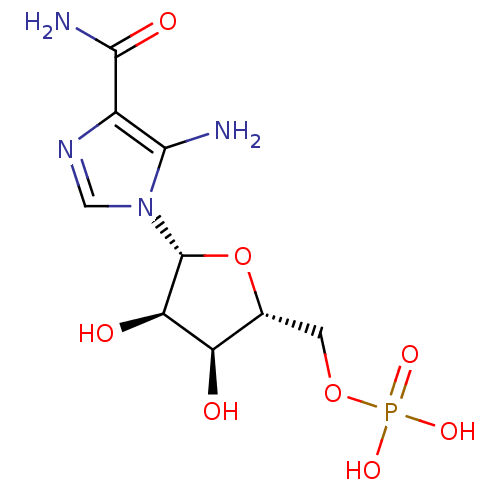
Substrate
Name:
BDBM22579
Synonyms:
AICAR | Aminoimidazole-4-carboxamide ribonucleotide | CHEMBL483849 | ZMP | {[(2R,3S,4R,5R)-5-(5-amino-4-carbamoyl-1H-imidazol-1-yl)-3,4-dihydroxyoxolan-2-yl]methoxy}phosphonic acid
Type:
Nucleoside or nucleotide
Emp. Form.:
C9H15N4O8P
Mol. Mass.:
338.2112
SMILES:
NC(=O)c1ncn([C@@H]2O[C@H](COP(O)(O)=O)[C@@H](O)[C@H]2O)c1N