Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
ATP phosphoribosyltransferase
Ligand
BDBM25325
Substrate
BDBM25315
Meas. Tech.
Enzyme Inhibition Assay
pH
8.5±n/a
Temperature
295.15±n/a K
Comments
15% inhibition @ 10 uM.
Citation
 Cho, Y; Ioerger, TR; Sacchettini, JC Discovery of novel nitrobenzothiazole inhibitors for Mycobacterium tuberculosis ATP phosphoribosyl transferase (HisG) through virtual screening. J Med Chem 51:5984-92 (2008) [PubMed] Article
Cho, Y; Ioerger, TR; Sacchettini, JC Discovery of novel nitrobenzothiazole inhibitors for Mycobacterium tuberculosis ATP phosphoribosyl transferase (HisG) through virtual screening. J Med Chem 51:5984-92 (2008) [PubMed] Article Target
Name:
ATP phosphoribosyltransferase
Synonyms:
ATP-PRT | ATP-PRTase | HIS1_MYCTU | hisG
Type:
Glycosyltransferase
Mol. Mass.:
30473.85
Organism:
Mycobacterium tuberculosis
Description:
n/a
Residue:
284
Sequence:
MLRVAVPNKGALSEPATEILAEAGYRRRTDSKDLTVIDPVNNVEFFFLRPKDIAIYVGSGELDFGITGRDLVCDSGAQVRERLALGFGSSSFRYAAPAGRNWTTADLAGMRIATAYPNLVRKDLATKGIEATVIRLDGAVEISVQLGVADAIADVVGSGRTLSQHDLVAFGEPLCDSEAVLIERAGTDGQDQTEARDQLVARVQGVVFGQQYLMLDYDCPRSALKKATAITPGLESPTIAPLADPDWVAIRALVPRRDVNGIMDELAAIGAKAILASDIRFCRF
Inhibitor
Name:
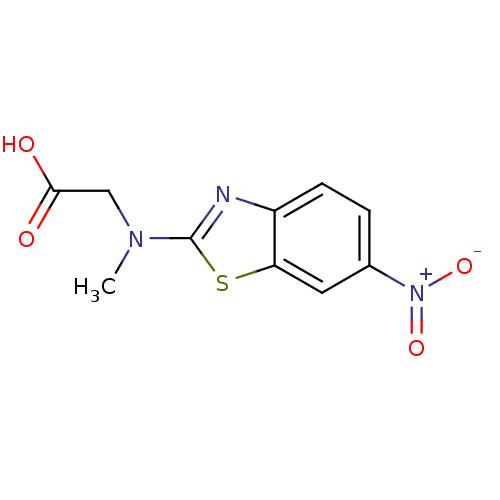
BDBM25325
Synonyms:
2-[methyl(6-nitro-1,3-benzothiazol-2-yl)amino]acetic acid | ChemBridge nitrobenzothiazole, 20
Type:
Small organic molecule
Emp. Form.:
C10H9N3O4S
Mol. Mass.:
267.261
SMILES:
CN(CC(O)=O)c1nc2ccc(cc2s1)[N+]([O-])=O
Substrate
Name:
BDBM25315
Synonyms:
Phosphoribosyl Pyrophosphate (PRPP) | {[(2R,3S,4R,5R)-3,4-dihydroxy-5-{[hydroxy(phosphonooxy)phosphoryl]oxy}oxolan-2-yl]methoxy}phosphonic acid
Type:
Pentosephosphate
Emp. Form.:
C5H13O14P3
Mol. Mass.:
390.0696
SMILES:
O[C@H]1[C@@H](O)[C@@H](OP(O)(=O)OP(O)(O)=O)O[C@@H]1COP(O)(O)=O |r|