Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Alpha-2A adrenergic receptor
Ligand
BDBM50001888
Substrate
n/a
Ki
64±n/a nM
Comments
PDSP_513
Citation
 O'Rourke, MF; Iversen, LJ; Lomasney, JW; Bylund, DB Species orthologs of the alpha-2A adrenergic receptor: the pharmacological properties of the bovine and rat receptors differ from the human and porcine receptors. J Pharmacol Exp Ther 271:735-40 (1994) [PubMed]
O'Rourke, MF; Iversen, LJ; Lomasney, JW; Bylund, DB Species orthologs of the alpha-2A adrenergic receptor: the pharmacological properties of the bovine and rat receptors differ from the human and porcine receptors. J Pharmacol Exp Ther 271:735-40 (1994) [PubMed] More Info.:
Target
Name:
Alpha-2A adrenergic receptor
Synonyms:
Alpha-2A adrenergic receptor | adrenergic Alpha2 | adrenergic Alpha2A
Type:
Enzyme Catalytic Domain
Mol. Mass.:
48998.96
Organism:
PIG
Description:
adrenergic Alpha2 0 PIG::P18871
Residue:
450
Sequence:
MGSLQPEAGNASWNGTEAPGGGARATPYSLQVTLTLVCLAGLLMLFTVFGNVLVIIAVFTSRALKAPQNLFLVSLASADILVATLVIPFSLANEVMGYWYFGKAWCEIYLALDVLFCTSSIVHLCAISLDRYWSITQAIEYNLKRTPRRIKAIIVTVWVISAVISFPPLISIEKKAGGGGQQPAEPRCEINDQKWYVISSCIGSFFAPCLIMILVYVRIYQIAKRRTRVPPSRRGPDAAAALPGGAERRPNGLGPERGVGRVGAEAEPLPVQLNGAPGEPAPAGPRDADGLDLEESSSSEHAERPPGPRRSERGPRAKSKARASQVKPGDSLPRRGPGAPGPGAPATGAGEERGGVAKASRWRGRQNREKRFTFVLAVVIGVFVVCWFPFFFTYTLTAVGCSVPPTLFKFFFWFGYCNSSLNPVIYTIFNHDFRRAFKKILCRGDRKRIV
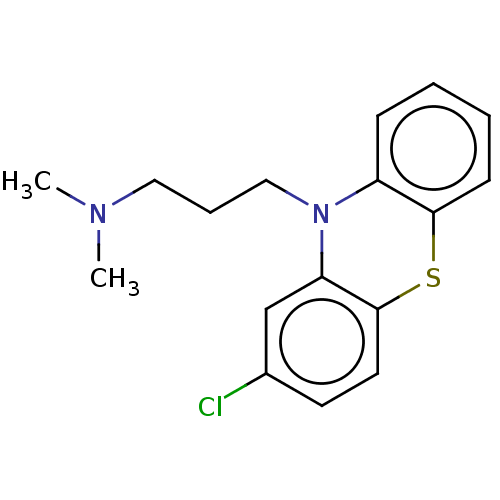
Inhibitor
Name:
BDBM50001888
Synonyms:
(chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)-propyl]-dimethyl-amine | (chlorpromazine)[3-(2-Chloro-phenothiazin-10-yl)-propyl]-dimethyl-amine | 1-(2-Allyl-phenoxy)-3-isopropylamino-propan-2-ol | 1N,1N-dimethyl-3-(2-chloro-10H-10-phenothiazinyl)-1-propanamine | 3-(2-chloro-10H-phenothiazin-10-yl)-N,N-dimethylpropan-1-amine | CHEMBL71 | CHLORPROMAZINE | CHLORPROMAZINE HIBENZATE | CHLORPROMAZINE HYDROCHLORIDE | CHLORPROMAZINE PHENOLPHTHALINATE | CHLORPROMAZINE TANNATE | Chlorpromazine;[3-(2-Chloro-phenothiazin-10-yl)-propyl]-dimethyl-amine | PROMAPAR | SONAZINE | THORAZINE | [3-(2-Chloro-phenothiazin-10-yl)-propyl]-dimethyl-amine (chlor-promazine) | [3-(2-Chloro-phenothiazin-10-yl)-propyl]-dimethyl-amine( Chlorpromazine) | [3-(2-Chloro-phenothiazin-10-yl)-propyl]-dimethyl-amine(clorpromazine) | chloropromazine | med.21724, Compound 15
Type:
Small organic molecule
Emp. Form.:
C17H19ClN2S
Mol. Mass.:
318.864
SMILES:
CN(C)CCCN1c2ccccc2Sc2ccc(Cl)cc12