Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Cyclin-dependent kinase 11B
Ligand
BDBM50466210
Substrate
n/a
Meas. Tech.
ChEMBL_1792696 (CHEMBL4264615)
IC50
>10000±n/a nM
Citation
 Wang, B; Wu, J; Wu, Y; Chen, C; Zou, F; Wang, A; Wu, H; Hu, Z; Jiang, Z; Liu, Q; Wang, W; Zhang, Y; Liu, F; Zhao, M; Hu, J; Huang, T; Ge, J; Wang, L; Ren, T; Wang, Y; Liu, J; Liu, Q Discovery of 4-(((4-(5-chloro-2-(((1s,4s)-4-((2-methoxyethyl)amino)cyclohexyl)amino)pyridin-4-yl)thiazol-2-yl)amino)methyl)tetrahydro-2H-pyran-4-carbonitrile (JSH-150) as a novel highly selective and potent CDK9 kinase inhibitor. Eur J Med Chem 158:896-916 (2018) [PubMed] Article
Wang, B; Wu, J; Wu, Y; Chen, C; Zou, F; Wang, A; Wu, H; Hu, Z; Jiang, Z; Liu, Q; Wang, W; Zhang, Y; Liu, F; Zhao, M; Hu, J; Huang, T; Ge, J; Wang, L; Ren, T; Wang, Y; Liu, J; Liu, Q Discovery of 4-(((4-(5-chloro-2-(((1s,4s)-4-((2-methoxyethyl)amino)cyclohexyl)amino)pyridin-4-yl)thiazol-2-yl)amino)methyl)tetrahydro-2H-pyran-4-carbonitrile (JSH-150) as a novel highly selective and potent CDK9 kinase inhibitor. Eur J Med Chem 158:896-916 (2018) [PubMed] Article More Info.:
Target
Name:
Cyclin-dependent kinase 11B
Synonyms:
2.7.11.22 | CD11B_HUMAN | CDC2L1 | CDK11 | CDK11B | CLK-1 | Cell division cycle 2-like protein kinase 1 | Cell division protein kinase 11B | Cyclin-dependent kinase | Cyclin-dependent kinase 11 (CDK11) | Cyclin-dependent kinase 11B | Galactosyltransferase-associated protein kinase p58/GTA | PITSLRE serine/threonine-protein kinase CDC2L1 | PITSLREA | PK58 | p58 CLK-1
Type:
Protein
Mol. Mass.:
92685.77
Organism:
Homo sapiens (Human)
Description:
P21127
Residue:
795
Sequence:
MGDEKDSWKVKTLDEILQEKKRRKEQEEKAEIKRLKNSDDRDSKRDSLEEGELRDHRMEITIRNSPYRREDSMEDRGEEDDSLAIKPPQQMSRKEKAHHRKDEKRKEKRRHRSHSAEGGKHARVKEKEREHERRKRHREEQDKARREWERQKRREMAREHSRRERDRLEQLERKRERERKMREQQKEQREQKERERRAEERRKEREARREVSAHHRTMREDYSDKVKASHWSRSPPRPPRERFELGDGRKPGEARPAPAQKPAQLKEEKMEERDLLSDLQDISDSERKTSSAESSSAESGSGSEEEEEEEEEEEEEGSTSEESEEEEEEEEEEEEETGSNSEEASEQSAEEVSEEEMSEDEERENENHLLVVPESRFDRDSGESEEAEEEVGEGTPQSSALTEGDYVPDSPALSPIELKQELPKYLPALQGCRSVEEFQCLNRIEEGTYGVVYRAKDKKTDEIVALKRLKMEKEKEGFPITSLREINTILKAQHPNIVTVREIVVGSNMDKIYIVMNYVEHDLKSLMETMKQPFLPGEVKTLMIQLLRGVKHLHDNWILHRDLKTSNLLLSHAGILKVGDFGLAREYGSPLKAYTPVVVTLWYRAPELLLGAKEYSTAVDMWSVGCIFGELLTQKPLFPGKSEIDQINKVFKDLGTPSEKIWPGYSELPAVKKMTFSEHPYNNLRKRFGALLSDQGFDLMNKFLTYFPGRRISAEDGLKHEYFRETPLPIDPSMFPTWPAKSEQQRVKRGTSPRPPEGGLGYSQLGDDDLKETGFHLTTTNQGASAAGPGFSLKF
Inhibitor
Name:
BDBM50466210
Synonyms:
CHEMBL4281048
Type:
Small organic molecule
Emp. Form.:
C24H33ClN6O2S
Mol. Mass.:
505.076
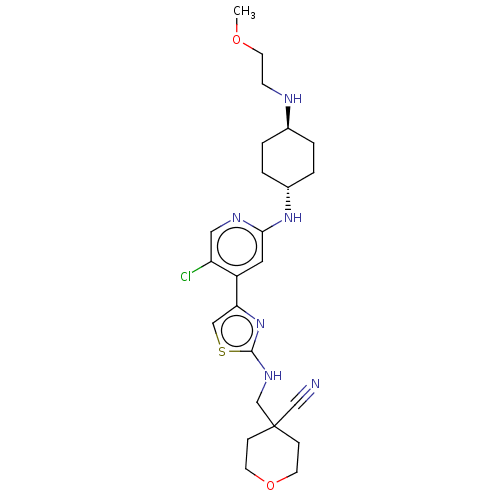
SMILES:
COCCN[C@H]1CC[C@@H](CC1)Nc1cc(-c2csc(NCC3(CCOCC3)C#N)n2)c(Cl)cn1 |r,wU:8.11,wD:5.4,(30.42,-38.05,;31.76,-37.28,;33.09,-38.05,;34.43,-37.28,;35.76,-38.05,;37.09,-37.28,;38.42,-38.05,;39.76,-37.28,;39.76,-35.74,;38.42,-34.97,;37.09,-35.74,;41.09,-34.97,;42.43,-35.74,;43.76,-34.97,;45.09,-35.74,;46.42,-34.97,;46.59,-33.43,;48.1,-33.12,;48.87,-34.45,;50.4,-34.45,;51.18,-35.78,;52.72,-35.77,;53.48,-37.11,;55.02,-37.11,;55.79,-35.77,;55.02,-34.44,;53.47,-34.43,;51.94,-37.1,;51.16,-38.43,;47.83,-35.59,;45.09,-37.28,;46.42,-38.05,;43.76,-38.05,;42.43,-37.28,)|