Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Retinoic acid receptor RXR-alpha
Ligand
BDBM50032219
Substrate
n/a
Meas. Tech.
ChEMBL_196496 (CHEMBL798310)
EC50
>10000±n/a nM
Citation
 Boehm, MF; McClurg, MR; Pathirana, C; Mangelsdorf, D; White, SK; Hebert, J; Winn, D; Goldman, ME; Heyman, RA Synthesis of high specific activity [3H]-9-cis-retinoic acid and its application for identifying retinoids with unusual binding properties. J Med Chem 37:408-14 (1994) [PubMed] Article
Boehm, MF; McClurg, MR; Pathirana, C; Mangelsdorf, D; White, SK; Hebert, J; Winn, D; Goldman, ME; Heyman, RA Synthesis of high specific activity [3H]-9-cis-retinoic acid and its application for identifying retinoids with unusual binding properties. J Med Chem 37:408-14 (1994) [PubMed] Article More Info.:
Target
Name:
Retinoic acid receptor RXR-alpha
Synonyms:
NR2B1 | Nuclear receptor subfamily 2 group B member 1 | Nuclear receptor subfamily 4 group A member 2 | Nuclear receptor subfamily 4 group A member 2/Retinoic acid receptor RXR-alpha | RXRA | RXRA_HUMAN | Retinoic acid receptor RXR-alpha/gamma | Retinoid X receptor alpha | Retinoid receptor
Type:
PROTEIN
Mol. Mass.:
50820.38
Organism:
Homo sapiens (Human)
Description:
ChEMBL_1456363
Residue:
462
Sequence:
MDTKHFLPLDFSTQVNSSLTSPTGRGSMAAPSLHPSLGPGIGSPGQLHSPISTLSSPINGMGPPFSVISSPMGPHSMSVPTTPTLGFSTGSPQLSSPMNPVSSSEDIKPPLGLNGVLKVPAHPSGNMASFTKHICAICGDRSSGKHYGVYSCEGCKGFFKRTVRKDLTYTCRDNKDCLIDKRQRNRCQYCRYQKCLAMGMKREAVQEERQRGKDRNENEVESTSSANEDMPVERILEAELAVEPKTETYVEANMGLNPSSPNDPVTNICQAADKQLFTLVEWAKRIPHFSELPLDDQVILLRAGWNELLIASFSHRSIAVKDGILLATGLHVHRNSAHSAGVGAIFDRVLTELVSKMRDMQMDKTELGCLRAIVLFNPDSKGLSNPAEVEALREKVYASLEAYCKHKYPEQPGRFAKLLLRLPALRSIGLKCLEHLFFFKLIGDTPIDTFLMEMLEAPHQMT
Inhibitor
Name:
BDBM50032219
Synonyms:
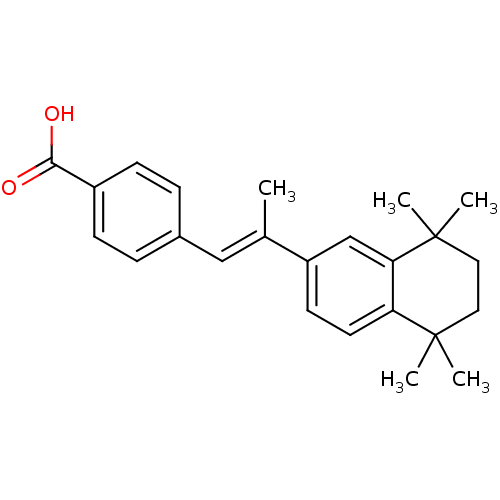
(E)-4-(2-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphthalen-2-yl)prop-1-enyl)benzoic acid | 4-(2-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphthalen-2-yl)prop-1-enyl)benzoic acid | 4-[(1E)-2-(5,5,8,8-TETRAMETHYL-5,6,7,8-TETRAHYDRONAPHTHALEN-2-YL)PROP-1-ENYL]BENZOIC ACID | 4-[(E)-2-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-naphthalen-2-yl)-propenyl]-benzoic acid | 4-[(E)-2-(5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthalenyl)-1-propenyl]benzoic acid | 4-[2-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-naphthalen-2-yl)-propenyl]-benzoic acid | CHEMBL275311 | US9963439, TTNPB
Type:
Small organic molecule
Emp. Form.:
C24H28O2
Mol. Mass.:
348.4779
SMILES:
C\C(=C/c1ccc(cc1)C(O)=O)c1ccc2c(c1)C(C)(C)CCC2(C)C