Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Glutamate receptor ionotropic, NMDA 1
Ligand
BDBM50078536
Substrate
n/a
Meas. Tech.
ChEMBL_141005 (CHEMBL747013)
Ki
3000±n/a nM
Citation
 Catarzi, D; Colotta, V; Varano, F; Cecchi, L; Filacchioni, G; Galli, A; Costagli, C 4,5-Dihydro-1,2,4-triazolo[1,5-a]quinoxalin-4-ones: excitatory amino acid antagonists with combined glycine/NMDA and AMPA receptor affinity. J Med Chem 42:2478-84 (1999) [PubMed] Article
Catarzi, D; Colotta, V; Varano, F; Cecchi, L; Filacchioni, G; Galli, A; Costagli, C 4,5-Dihydro-1,2,4-triazolo[1,5-a]quinoxalin-4-ones: excitatory amino acid antagonists with combined glycine/NMDA and AMPA receptor affinity. J Med Chem 42:2478-84 (1999) [PubMed] Article More Info.:
Target
Name:
Glutamate receptor ionotropic, NMDA 1
Synonyms:
Glutamate (NMDA) receptor subunit zeta 1 | Glutamate [NMDA] receptor subunit zeta-1 | Glutamate-NMDA-Channel | Glutamate-NMDA-MK801 | Glutamate-NMDA-Polyamine | Grin1 | NMDA | NMDZ1_RAT | Nmdar1 | phencyclidine
Type:
Enzyme Catalytic Domain
Mol. Mass.:
105533.40
Organism:
RAT
Description:
P35439
Residue:
938
Sequence:
MSTMHLLTFALLFSCSFARAACDPKIVNIGAVLSTRKHEQMFREAVNQANKRHGSWKIQLNATSVTHKPNAIQMALSVCEDLISSQVYAILVSHPPTPNDHFTPTPVSYTAGFYRIPVLGLTTRMSIYSDKSIHLSFLRTVPPYSHQSSVWFEMMRVYNWNHIILLVSDDHEGRAAQKRLETLLEERESKAEKVLQFDPGTKNVTALLMEARELEARVIILSASEDDAATVYRAAAMLNMTGSGYVWLVGEREISGNALRYAPDGIIGLQLINGKNESAHISDAVGVVAQAVHELLEKENITDPPRGCVGNTNIWKTGPLFKRVLMSSKYADGVTGRVEFNEDGDRKFANYSIMNLQNRKLVQVGIYNGTHVIPNDRKIIWPGGETEKPRGYQMSTRLKIVTIHQEPFVYVKPTMSDGTCKEEFTVNGDPVKKVICTGPNDTSPGSPRHTVPQCCYGFCIDLLIKLARTMNFTYEVHLVADGKFGTQERVNNSNKKEWNGMMGELLSGQADMIVAPLTINNERAQYIEFSKPFKYQGLTILVKKEIPRSTLDSFMQPFQSTLWLLVGLSVHVVAVMLYLLDRFSPFGRFKVNSEEEEEDALTLSSAMWFSWGVLLNSGIGEGAPRSFSARILGMVWAGFAMIIVASYTANLAAFLVLDRPEERITGINDPRLRNPSDKFIYATVKQSSVDIYFRRQVELSTMYRHMEKHNYESAAEAIQAVRDNKLHAFIWDSAVLEFEASQKCDLVTTGELFFRSGFGIGMRKDSPWKQNVSLSILKSHENGFMEDLDKTWVRYQECDSRSNAPATLTFENMAGVFMLVAGGIVAGIFLIFIEIAYKRHKDARRKQMQLAFAAVNVWRKNLQDRKSGRAEPDPKKKATFRAITSTLASSFKRRRSSKDTSTGGGRGALQNQKDTVLPRRAIEREEGQLQLCSRHRES
Inhibitor
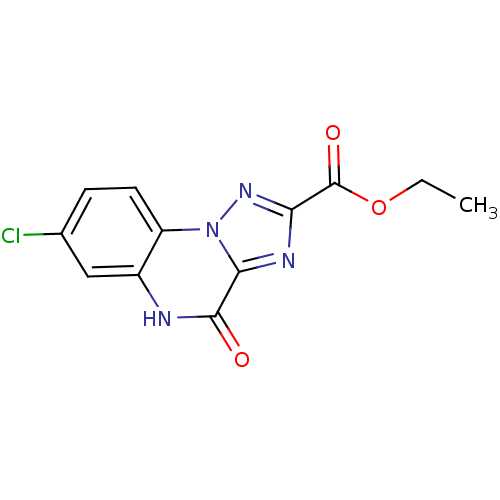
Name:
BDBM50078536
Synonyms:
7-Chloro-4-oxo-4,5-dihydro-[1,2,4]triazolo[1,5-a]quinoxaline-2-carboxylic acid ethyl ester | CHEMBL83919 | ethyl 7-chloro-4-oxo-4,5-dihydro-[1,2,4]triazolo[1,5-a]quinoxaline-2-carboxylate
Type:
Small organic molecule
Emp. Form.:
C12H9ClN4O3
Mol. Mass.:
292.678
SMILES:
CCOC(=O)c1nc2n(n1)c1ccc(Cl)cc1[nH]c2=O