Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Proprotein convertase subtilisin/kexin type 9
Ligand
BDBM50554734
Substrate
n/a
Meas. Tech.
ChEMBL_2050524 (CHEMBL4705223)
Ki
1.5±n/a nM
Citation
 Alleyne, C; Amin, RP; Bhatt, B; Bianchi, E; Blain, JC; Boyer, N; Branca, D; Embrey, MW; Ha, SN; Jette, K; Johns, DG; Kerekes, AD; Koeplinger, KA; LaPlaca, D; Li, N; Murphy, B; Orth, P; Ricardo, A; Salowe, S; Seyb, K; Shahripour, A; Stringer, JR; Sun, Y; Tracy, R; Wu, C; Xiong, Y; Youm, H; Zokian, HJ; Tucker, TJ Series of Novel and Highly Potent Cyclic Peptide PCSK9 Inhibitors Derived from an mRNA Display Screen and Optimized via Structure-Based Design. J Med Chem 63:13796-13824 (2020) [PubMed] Article
Alleyne, C; Amin, RP; Bhatt, B; Bianchi, E; Blain, JC; Boyer, N; Branca, D; Embrey, MW; Ha, SN; Jette, K; Johns, DG; Kerekes, AD; Koeplinger, KA; LaPlaca, D; Li, N; Murphy, B; Orth, P; Ricardo, A; Salowe, S; Seyb, K; Shahripour, A; Stringer, JR; Sun, Y; Tracy, R; Wu, C; Xiong, Y; Youm, H; Zokian, HJ; Tucker, TJ Series of Novel and Highly Potent Cyclic Peptide PCSK9 Inhibitors Derived from an mRNA Display Screen and Optimized via Structure-Based Design. J Med Chem 63:13796-13824 (2020) [PubMed] Article More Info.:
Target
Name:
Proprotein convertase subtilisin/kexin type 9
Synonyms:
NARC-1 | NARC1 | Neural apoptosis-regulated convertase 1 | PC9 | PCSK9 | PCSK9_HUMAN | Proprotein convertase 9 | Proprotein convertase subtilisin/kexin type 9 | Proprotein convertase subtilisin/kexin type 9 (PCSK9) | Subtilisin/kexin type 9 | Subtilisin/kexin-like protease PC9
Type:
Enzyme
Mol. Mass.:
74286.93
Organism:
Homo sapiens (Human)
Description:
Q8NBP7
Residue:
692
Sequence:
MGTVSSRRSWWPLPLLLLLLLLLGPAGARAQEDEDGDYEELVLALRSEEDGLAEAPEHGTTATFHRCAKDPWRLPGTYVVVLKEETHLSQSERTARRLQAQAARRGYLTKILHVFHGLLPGFLVKMSGDLLELALKLPHVDYIEEDSSVFAQSIPWNLERITPPRYRADEYQPPDGGSLVEVYLLDTSIQSDHREIEGRVMVTDFENVPEEDGTRFHRQASKCDSHGTHLAGVVSGRDAGVAKGASMRSLRVLNCQGKGTVSGTLIGLEFIRKSQLVQPVGPLVVLLPLAGGYSRVLNAACQRLARAGVVLVTAAGNFRDDACLYSPASAPEVITVGATNAQDQPVTLGTLGTNFGRCVDLFAPGEDIIGASSDCSTCFVSQSGTSQAAAHVAGIAAMMLSAEPELTLAELRQRLIHFSAKDVINEAWFPEDQRVLTPNLVAALPPSTHGAGWQLFCRTVWSAHSGPTRMATAVARCAPDEELLSCSSFSRSGKRRGERMEAQGGKLVCRAHNAFGGEGVYAIARCCLLPQANCSVHTAPPAEASMGTRVHCHQQGHVLTGCSSHWEVEDLGTHKPPVLRPRGQPNQCVGHREASIHASCCHAPGLECKVKEHGIPAPQEQVTVACEEGWTLTGCSALPGTSHVLGAYAVDNTCVVRSRDVSTTGSTSEGAVTAVAICCRSRHLAQASQELQ
Inhibitor
Name:
BDBM50554734
Synonyms:
CHEMBL4761415 | US11530244, Compound 289
Type:
Small organic molecule
Emp. Form.:
C69H81F3N14O12S2
Mol. Mass.:
1419.593
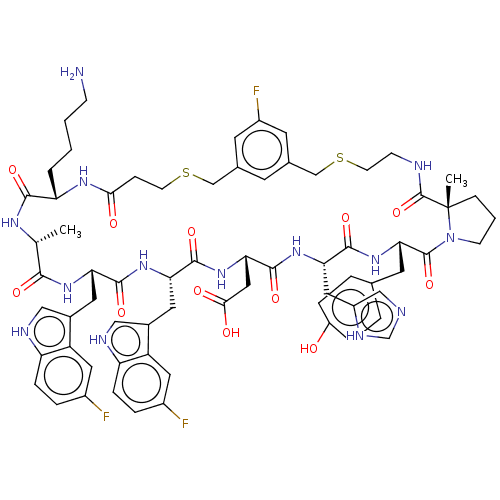
SMILES:
C[C@H]1NC(=O)[C@H](CCCCN)NC(=O)CCSCc2cc(F)cc(CSCCNC(=O)[C@]3(C)CCCN3C(=O)[C@H](Cc3ccc(O)cc3)NC(=O)[C@H](Cc3cnc[nH]3)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc3c[nH]c4ccc(F)cc34)NC(=O)[C@H](Cc3c[nH]c4ccc(F)cc34)NC1=O)c2 |r|