Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Monoglyceride lipase
Ligand
BDBM50566998
Substrate
n/a
Meas. Tech.
ChEMBL_2102182 (CHEMBL4810578)
IC50
5.4±n/a nM
Citation
 Ikeda, S; Sugiyama, H; Tokuhara, H; Murakami, M; Nakamura, M; Oguro, Y; Aida, J; Morishita, N; Sogabe, S; Dougan, DR; Gay, SC; Qin, L; Arimura, N; Takahashi, Y; Sasaki, M; Kamada, Y; Aoyama, K; Kimoto, K; Kamata, M Design and Synthesis of Novel Spiro Derivatives as Potent and Reversible Monoacylglycerol Lipase (MAGL) Inhibitors: Bioisosteric Transformation from 3-Oxo-3,4-dihydro-2 J Med Chem 64:11014-11044 (2021) [PubMed] Article
Ikeda, S; Sugiyama, H; Tokuhara, H; Murakami, M; Nakamura, M; Oguro, Y; Aida, J; Morishita, N; Sogabe, S; Dougan, DR; Gay, SC; Qin, L; Arimura, N; Takahashi, Y; Sasaki, M; Kamada, Y; Aoyama, K; Kimoto, K; Kamata, M Design and Synthesis of Novel Spiro Derivatives as Potent and Reversible Monoacylglycerol Lipase (MAGL) Inhibitors: Bioisosteric Transformation from 3-Oxo-3,4-dihydro-2 J Med Chem 64:11014-11044 (2021) [PubMed] Article More Info.:
Target
Name:
Monoglyceride lipase
Synonyms:
HU-K5 | Lysophospholipase homolog | Lysophospholipase-like | MAGL | MGL | MGLL | MGLL_HUMAN
Type:
Hydrolase
Mol. Mass.:
33264.56
Organism:
Homo sapiens (Human)
Description:
Human recombinant MGL (Cayman Chemical, cat# 10008354).
Residue:
303
Sequence:
MPEESSPRRTPQSIPYQDLPHLVNADGQYLFCRYWKPTGTPKALIFVSHGAGEHSGRYEELARMLMGLDLLVFAHDHVGHGQSEGERMVVSDFHVFVRDVLQHVDSMQKDYPGLPVFLLGHSMGGAIAILTAAERPGHFAGMVLISPLVLANPESATTFKVLAAKVLNLVLPNLSLGPIDSSVLSRNKTEVDIYNSDPLICRAGLKVCFGIQLLNAVSRVERALPKLTVPFLLLQGSADRLCDSKGAYLLMELAKSQDKTLKIYEGAYHVLHKELPEVTNSVFHEINMWVSQRTATAGTASPP
Inhibitor
Name:
BDBM50566998
Synonyms:
CHEMBL4865443
Type:
Small organic molecule
Emp. Form.:
C18H18ClF3N2O4
Mol. Mass.:
418.795
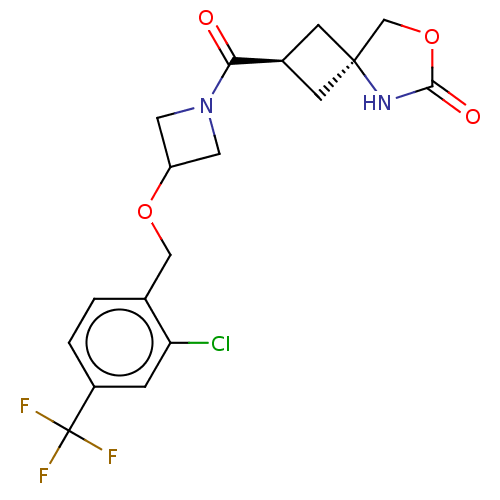
SMILES:
FC(F)(F)c1ccc(COC2CN(C2)C(=O)[C@H]2C[C@@]3(C2)COC(=O)N3)c(Cl)c1 |r,wD:16.16,18.26,(.93,-8.56,;2.26,-9.33,;2.26,-10.87,;.92,-10.09,;3.59,-8.56,;4.93,-9.34,;6.27,-8.57,;6.26,-7.03,;7.6,-6.27,;8.93,-7.04,;10.27,-6.28,;10.66,-4.79,;12.15,-5.2,;11.75,-6.68,;13.48,-4.43,;13.48,-2.89,;14.82,-5.2,;15.21,-6.68,;16.69,-6.28,;16.3,-4.79,;16.68,-7.82,;18.15,-8.3,;19.06,-7.06,;20.6,-7.07,;18.16,-5.81,;4.94,-6.26,;4.94,-4.72,;3.6,-7.02,)|