Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Glycogen phosphorylase, liver form
Ligand
BDBM50065965
Substrate
n/a
Meas. Tech.
ChEMBL_304931 (CHEMBL826961)
IC50
110±n/a nM
Citation
 Wright, SW; Rath, VL; Genereux, PE; Hageman, DL; Levy, CB; McClure, LD; McCoid, SC; McPherson, RK; Schelhorn, TM; Wilder, DE; Zavadoski, WJ; Gibbs, EM; Treadway, JL 5-Chloroindoloyl glycine amide inhibitors of glycogen phosphorylase: synthesis, in vitro, in vivo, and X-ray crystallographic characterization. Bioorg Med Chem Lett 15:459-65 (2004) [PubMed] Article
Wright, SW; Rath, VL; Genereux, PE; Hageman, DL; Levy, CB; McClure, LD; McCoid, SC; McPherson, RK; Schelhorn, TM; Wilder, DE; Zavadoski, WJ; Gibbs, EM; Treadway, JL 5-Chloroindoloyl glycine amide inhibitors of glycogen phosphorylase: synthesis, in vitro, in vivo, and X-ray crystallographic characterization. Bioorg Med Chem Lett 15:459-65 (2004) [PubMed] Article More Info.:
Target
Name:
Glycogen phosphorylase, liver form
Synonyms:
Glycogen Phosphorylase (PYGL) | Glycogen Phosphorylase, liver form | Liver glycogen phosphorylase | PYGL | PYGL_HUMAN
Type:
Homodimer
Mol. Mass.:
97153.98
Organism:
Homo sapiens (Human)
Description:
Dimers associate into a tetramer to form the enzymatically active phosphorylase A.
Residue:
847
Sequence:
MAKPLTDQEKRRQISIRGIVGVENVAELKKSFNRHLHFTLVKDRNVATTRDYYFALAHTVRDHLVGRWIRTQQHYYDKCPKRVYYLSLEFYMGRTLQNTMINLGLQNACDEAIYQLGLDIEELEEIEEDAGLGNGGLGRLAACFLDSMATLGLAAYGYGIRYEYGIFNQKIRDGWQVEEADDWLRYGNPWEKSRPEFMLPVHFYGKVEHTNTGTKWIDTQVVLALPYDTPVPGYMNNTVNTMRLWSARAPNDFNLRDFNVGDYIQAVLDRNLAENISRVLYPNDNFFEGKELRLKQEYFVVAATLQDIIRRFKASKFGSTRGAGTVFDAFPDQVAIQLNDTHPALAIPELMRIFVDIEKLPWSKAWELTQKTFAYTNHTVLPEALERWPVDLVEKLLPRHLEIIYEINQKHLDRIVALFPKDVDRLRRMSLIEEEGSKRINMAHLCIVGSHAVNGVAKIHSDIVKTKVFKDFSELEPDKFQNKTNGITPRRWLLLCNPGLAELIAEKIGEDYVKDLSQLTKLHSFLGDDVFLRELAKVKQENKLKFSQFLETEYKVKINPSSMFDVQVKRIHEYKRQLLNCLHVITMYNRIKKDPKKLFVPRTVIIGGKAAPGYHMAKMIIKLITSVADVVNNDPMVGSKLKVIFLENYRVSLAEKVIPATDLSEQISTAGTEASGTGNMKFMLNGALTIGTMDGANVEMAEEAGEENLFIFGMRIDDVAALDKKGYEAKEYYEALPELKLVIDQIDNGFFSPKQPDLFKDIINMLFYHDRFKVFADYEAYVKCQDKVSQLYMNPKAWNTMVLKNIAASGKFSSDRTIKEYAQNIWNVEPSDLKISLSNESNKVNGN
Inhibitor
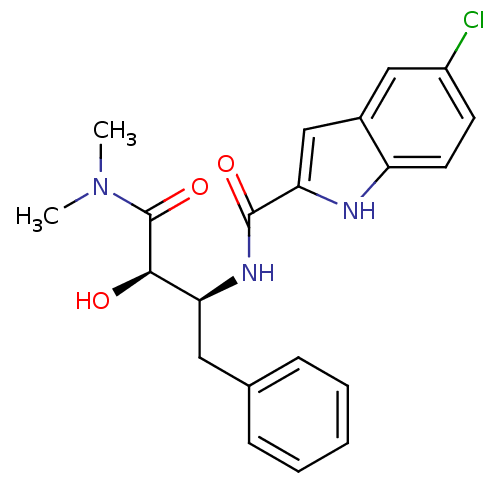
Name:
BDBM50065965
Synonyms:
5-Chloro-1H-indole-2-carboxylic acid ((1S,2R)-1-benzyl-2-dimethylcarbamoyl-2-hydroxy-ethyl)-amide | 5-Chloro-1H-indole-2-carboxylic acid [2-((R)-dimethyl-carbamoyl)-2-hydroxy-1-((S)-phenylmethyl)-ethyl]-amide | CHEMBL319136 | CP-91149
Type:
Small organic molecule
Emp. Form.:
C21H22ClN3O3
Mol. Mass.:
399.871
SMILES:
CN(C)C(=O)[C@H](O)[C@H](Cc1ccccc1)NC(=O)c1cc2cc(Cl)ccc2[nH]1