Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Peroxisome proliferator-activated receptor gamma
Ligand
BDBM50193729
Substrate
n/a
Meas. Tech.
ChEMBL_424080 (CHEMBL855083)
IC50
60±n/a nM
Citation
 Hopkins, CR; O'neil, SV; Laufersweiler, MC; Wang, Y; Pokross, M; Mekel, M; Evdokimov, A; Walter, R; Kontoyianni, M; Petrey, ME; Sabatakos, G; Roesgen, JT; Richardson, E; Demuth, TP Design and synthesis of novel N-sulfonyl-2-indole carboxamides as potent PPAR-gamma binding agents with potential application to the treatment of osteoporosis. Bioorg Med Chem Lett 16:5659-63 (2006) [PubMed] Article
Hopkins, CR; O'neil, SV; Laufersweiler, MC; Wang, Y; Pokross, M; Mekel, M; Evdokimov, A; Walter, R; Kontoyianni, M; Petrey, ME; Sabatakos, G; Roesgen, JT; Richardson, E; Demuth, TP Design and synthesis of novel N-sulfonyl-2-indole carboxamides as potent PPAR-gamma binding agents with potential application to the treatment of osteoporosis. Bioorg Med Chem Lett 16:5659-63 (2006) [PubMed] Article More Info.:
Target
Name:
Peroxisome proliferator-activated receptor gamma
Synonyms:
NR1C3 | Nuclear receptor subfamily 1 group C member 3 | PPAR-gamma | PPARG | PPARG_HUMAN | Peroxisome proliferator-activated receptor | Peroxisome proliferator-activated receptor gamma (PPAR gamma) | Peroxisome proliferator-activated receptor gamma (PPARG) | Peroxisome proliferator-activated receptor gamma (PPARγ) | Peroxisome proliferator-activated receptor gamma/Nuclear receptor corepressor 2 | peroxisome proliferator-activated receptor gamma isoform 2
Type:
Nuclear Receptor
Mol. Mass.:
57613.46
Organism:
Homo sapiens (Human)
Description:
P37231
Residue:
505
Sequence:
MGETLGDSPIDPESDSFTDTLSANISQEMTMVDTEMPFWPTNFGISSVDLSVMEDHSHSFDIKPFTTVDFSSISTPHYEDIPFTRTDPVVADYKYDLKLQEYQSAIKVEPASPPYYSEKTQLYNKPHEEPSNSLMAIECRVCGDKASGFHYGVHACEGCKGFFRRTIRLKLIYDRCDLNCRIHKKSRNKCQYCRFQKCLAVGMSHNAIRFGRMPQAEKEKLLAEISSDIDQLNPESADLRALAKHLYDSYIKSFPLTKAKARAILTGKTTDKSPFVIYDMNSLMMGEDKIKFKHITPLQEQSKEVAIRIFQGCQFRSVEAVQEITEYAKSIPGFVNLDLNDQVTLLKYGVHEIIYTMLASLMNKDGVLISEGQGFMTREFLKSLRKPFGDFMEPKFEFAVKFNALELDDSDLAIFIAVIILSGDRPGLLNVKPIEDIQDNLLQALELQLKLNHPESSQLFAKLLQKMTDLRQIVTEHVQLLQVIKKTETDMSLHPLLQEIYKDLY
Inhibitor
Name:
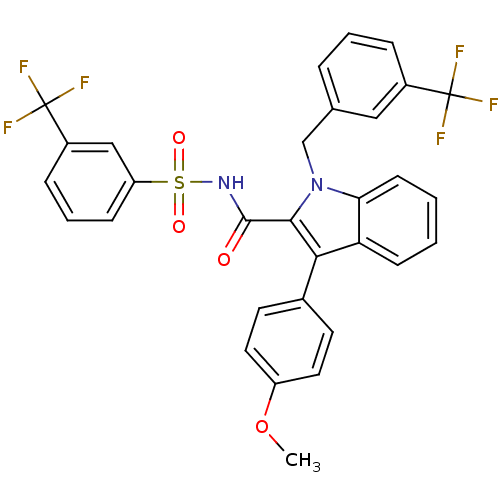
BDBM50193729
Synonyms:
1-(3-(trifluoromethyl)benzyl)-3-(4-methoxyphenyl)-N-(3-(trifluoromethyl)phenylsulfonyl)-1H-indole-2-carboxamide | CHEMBL267881
Type:
Small organic molecule
Emp. Form.:
C31H22F6N2O4S
Mol. Mass.:
632.573
SMILES:
COc1ccc(cc1)-c1c(C(=O)NS(=O)(=O)c2cccc(c2)C(F)(F)F)n(Cc2cccc(c2)C(F)(F)F)c2ccccc12