Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Cytochrome P450 1A2
Ligand
BDBM50246354
Substrate
n/a
Meas. Tech.
ChEMBL_559140 (CHEMBL1012085)
IC50
>40000±n/a nM
Citation
 Xia, M; Hou, C; DeMong, D; Pollack, S; Pan, M; Singer, M; Matheis, M; Murray, W; Cavender, D; Wachter, M Synthesis and structure-activity relationship of 7-azaindole piperidine derivatives as CCR2 antagonists. Bioorg Med Chem Lett 18:6468-70 (2008) [PubMed] Article
Xia, M; Hou, C; DeMong, D; Pollack, S; Pan, M; Singer, M; Matheis, M; Murray, W; Cavender, D; Wachter, M Synthesis and structure-activity relationship of 7-azaindole piperidine derivatives as CCR2 antagonists. Bioorg Med Chem Lett 18:6468-70 (2008) [PubMed] Article More Info.:
Target
Name:
Cytochrome P450 1A2
Synonyms:
CP1A2_HUMAN | CYP1A2 | CYPIA2 | Cholesterol 25-hydroxylase | Cytochrome P(3)450 | Cytochrome P450 1A | Cytochrome P450 1A2 (CYP1A2) | Cytochrome P450 4 | Cytochrome P450-P3
Type:
Enzyme
Mol. Mass.:
58423.38
Organism:
Homo sapiens (Human)
Description:
P05177
Residue:
516
Sequence:
MALSQSVPFSATELLLASAIFCLVFWVLKGLRPRVPKGLKSPPEPWGWPLLGHVLTLGKNPHLALSRMSQRYGDVLQIRIGSTPVLVLSRLDTIRQALVRQGDDFKGRPDLYTSTLITDGQSLTFSTDSGPVWAARRRLAQNALNTFSIASDPASSSSCYLEEHVSKEAKALISRLQELMAGPGHFDPYNQVVVSVANVIGAMCFGQHFPESSDEMLSLVKNTHEFVETASSGNPLDFFPILRYLPNPALQRFKAFNQRFLWFLQKTVQEHYQDFDKNSVRDITGALFKHSKKGPRASGNLIPQEKIVNLVNDIFGAGFDTVTTAISWSLMYLVTKPEIQRKIQKELDTVIGRERRPRLSDRPQLPYLEAFILETFRHSSFLPFTIPHSTTRDTTLNGFYIPKKCCVFVNQWQVNHDPELWEDPSEFRPERFLTADGTAINKPLSEKMMLFGMGKRRCIGEVLAKWEIFLFLAILLQQLEFSVPPGVKVDLTPIYGLTMKHARCEHVQARLRFSIN
Inhibitor
Name:
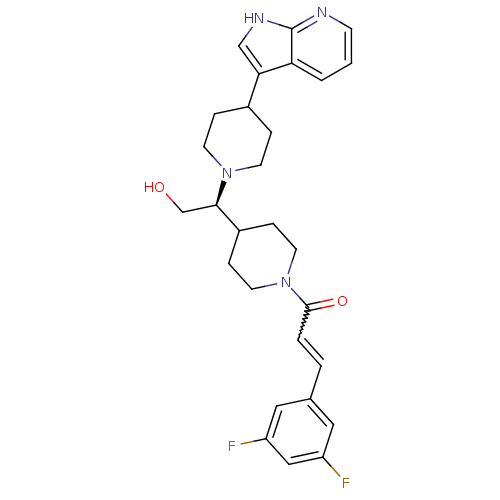
BDBM50246354
Synonyms:
(S)-1-(4-(1-(4-(1H-pyrrolo[2,3-b]pyridin-3-yl)piperidin-1-yl)-2-hydroxyethyl)piperidin-1-yl)-3-(3,5-difluorophenyl)prop-2-en-1-one | CHEMBL455683
Type:
Small organic molecule
Emp. Form.:
C28H32F2N4O2
Mol. Mass.:
494.5761
SMILES:
OC[C@H](C1CCN(CC1)C(=O)C=Cc1cc(F)cc(F)c1)N1CCC(CC1)c1c[nH]c2ncccc12 |r,w:11.11|