Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Cytochrome P450 3A4
Ligand
BDBM19188
Substrate
n/a
Meas. Tech.
ChEMBL_590750 (CHEMBL1050892)
IC50
5000±n/a nM
Citation
 Pisani, L; Muncipinto, G; Miscioscia, TF; Nicolotti, O; Leonetti, F; Catto, M; Caccia, C; Salvati, P; Soto-Otero, R; Mendez-Alvarez, E; Passeleu, C; Carotti, A Discovery of a novel class of potent coumarin monoamine oxidase B inhibitors: development and biopharmacological profiling of 7-[(3-chlorobenzyl)oxy]-4-[(methylamino)methyl]-2H-chromen-2-one methanesulfonate (NW-1772) as a highly potent, selective, reversible, and orally active monoamine oxidase B J Med Chem 52:6685-706 (2009) [PubMed] Article
Pisani, L; Muncipinto, G; Miscioscia, TF; Nicolotti, O; Leonetti, F; Catto, M; Caccia, C; Salvati, P; Soto-Otero, R; Mendez-Alvarez, E; Passeleu, C; Carotti, A Discovery of a novel class of potent coumarin monoamine oxidase B inhibitors: development and biopharmacological profiling of 7-[(3-chlorobenzyl)oxy]-4-[(methylamino)methyl]-2H-chromen-2-one methanesulfonate (NW-1772) as a highly potent, selective, reversible, and orally active monoamine oxidase B J Med Chem 52:6685-706 (2009) [PubMed] Article More Info.:
Target
Name:
Cytochrome P450 3A4
Synonyms:
Albendazole monooxygenase | Albendazole sulfoxidase | CP3A4_HUMAN | CYP3A3 | CYP3A4 | CYPIIIA3 | CYPIIIA4 | Cytochrome P450 3A3 | Cytochrome P450 3A4 (CYP3A4) | Cytochrome P450 HLp | Nifedipine oxidase | Quinine 3-monooxygenase | Taurochenodeoxycholate 6-alpha-hydroxylase
Type:
Enzyme
Mol. Mass.:
57349.57
Organism:
Homo sapiens (Human)
Description:
n/a
Residue:
503
Sequence:
MALIPDLAMETWLLLAVSLVLLYLYGTHSHGLFKKLGIPGPTPLPFLGNILSYHKGFCMFDMECHKKYGKVWGFYDGQQPVLAITDPDMIKTVLVKECYSVFTNRRPFGPVGFMKSAISIAEDEEWKRLRSLLSPTFTSGKLKEMVPIIAQYGDVLVRNLRREAETGKPVTLKDVFGAYSMDVITSTSFGVNIDSLNNPQDPFVENTKKLLRFDFLDPFFLSITVFPFLIPILEVLNICVFPREVTNFLRKSVKRMKESRLEDTQKHRVDFLQLMIDSQNSKETESHKALSDLELVAQSIIFIFAGYETTSSVLSFIMYELATHPDVQQKLQEEIDAVLPNKAPPTYDTVLQMEYLDMVVNETLRLFPIAMRLERVCKKDVEINGMFIPKGVVVMIPSYALHRDPKYWTEPEKFLPERFSKKNKDNIDPYIYTPFGSGPRNCIGMRFALMNMKLALIRVLQNFSFKPCKETQIPLKLSLGGLLQPEKPVVLKVESRDGTVSGA
Inhibitor
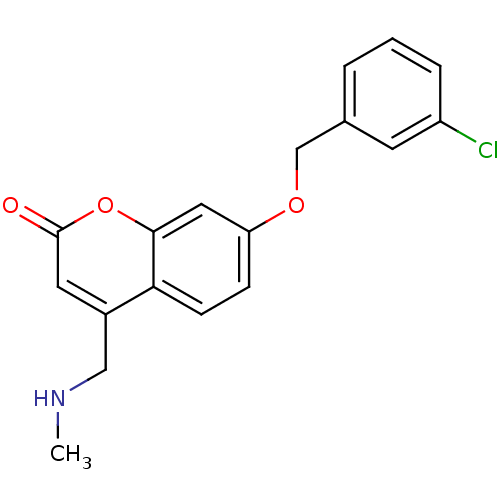
Name:
BDBM19188
Synonyms:
7-(3-chlorobenzyloxy)-4-(methylamino)methyl-coumarin, 2 | 7-[(3-chlorophenyl)methoxy]-4-[(methylamino)methyl]-2H-chromen-2-one | C18 | CHEMBL593763
Type:
Small organic molecule
Emp. Form.:
C18H16ClNO3
Mol. Mass.:
329.778
SMILES:
CNCc1cc(=O)oc2cc(OCc3cccc(Cl)c3)ccc12