Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Cytochrome P450 3A4
Ligand
BDBM50326132
Substrate
n/a
Meas. Tech.
ChEMBL_657985 (CHEMBL1248766)
IC50
5000±n/a nM
Citation
 Bromidge, SM; Arban, R; Bertani, B; Bison, S; Borriello, M; Cavanni, P; Dal Forno, G; Di-Fabio, R; Donati, D; Fontana, S; Gianotti, M; Gordon, LJ; Granci, E; Leslie, CP; Moccia, L; Pasquarello, A; Sartori, I; Sava, A; Watson, JM; Worby, A; Zonzini, L; Zucchelli, V Design and synthesis of novel tricyclic benzoxazines as potent 5-HT(1A/B/D) receptor antagonists leading to the discovery of 6-{2-[4-(2-methyl-5-quinolinyl)-1-piperazinyl]ethyl}-4H-imidazo[5,1-c][1,4]benzoxazine-3-carboxamide (GSK588045). J Med Chem 53:5827-43 (2010) [PubMed] Article
Bromidge, SM; Arban, R; Bertani, B; Bison, S; Borriello, M; Cavanni, P; Dal Forno, G; Di-Fabio, R; Donati, D; Fontana, S; Gianotti, M; Gordon, LJ; Granci, E; Leslie, CP; Moccia, L; Pasquarello, A; Sartori, I; Sava, A; Watson, JM; Worby, A; Zonzini, L; Zucchelli, V Design and synthesis of novel tricyclic benzoxazines as potent 5-HT(1A/B/D) receptor antagonists leading to the discovery of 6-{2-[4-(2-methyl-5-quinolinyl)-1-piperazinyl]ethyl}-4H-imidazo[5,1-c][1,4]benzoxazine-3-carboxamide (GSK588045). J Med Chem 53:5827-43 (2010) [PubMed] Article More Info.:
Target
Name:
Cytochrome P450 3A4
Synonyms:
Albendazole monooxygenase | Albendazole sulfoxidase | CP3A4_HUMAN | CYP3A3 | CYP3A4 | CYPIIIA3 | CYPIIIA4 | Cytochrome P450 3A3 | Cytochrome P450 3A4 (CYP3A4) | Cytochrome P450 HLp | Nifedipine oxidase | Quinine 3-monooxygenase | Taurochenodeoxycholate 6-alpha-hydroxylase
Type:
Enzyme
Mol. Mass.:
57349.57
Organism:
Homo sapiens (Human)
Description:
n/a
Residue:
503
Sequence:
MALIPDLAMETWLLLAVSLVLLYLYGTHSHGLFKKLGIPGPTPLPFLGNILSYHKGFCMFDMECHKKYGKVWGFYDGQQPVLAITDPDMIKTVLVKECYSVFTNRRPFGPVGFMKSAISIAEDEEWKRLRSLLSPTFTSGKLKEMVPIIAQYGDVLVRNLRREAETGKPVTLKDVFGAYSMDVITSTSFGVNIDSLNNPQDPFVENTKKLLRFDFLDPFFLSITVFPFLIPILEVLNICVFPREVTNFLRKSVKRMKESRLEDTQKHRVDFLQLMIDSQNSKETESHKALSDLELVAQSIIFIFAGYETTSSVLSFIMYELATHPDVQQKLQEEIDAVLPNKAPPTYDTVLQMEYLDMVVNETLRLFPIAMRLERVCKKDVEINGMFIPKGVVVMIPSYALHRDPKYWTEPEKFLPERFSKKNKDNIDPYIYTPFGSGPRNCIGMRFALMNMKLALIRVLQNFSFKPCKETQIPLKLSLGGLLQPEKPVVLKVESRDGTVSGA
Inhibitor
Name:
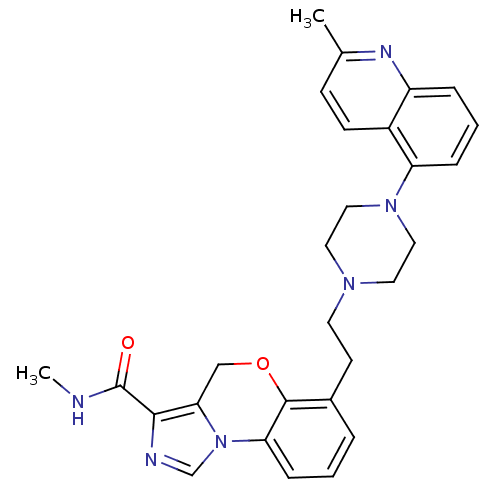
BDBM50326132
Synonyms:
CHEMBL1241912 | N-methyl-6-(2-(4-(2-methylquinolin-5-yl)piperazin-1-yl)ethyl)-4H-benzo[b]imidazo[1,5-d][1,4]oxazine-3-carboxamide
Type:
Small organic molecule
Emp. Form.:
C28H30N6O2
Mol. Mass.:
482.5768
SMILES:
CNC(=O)c1ncn-2c1COc1c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc-21