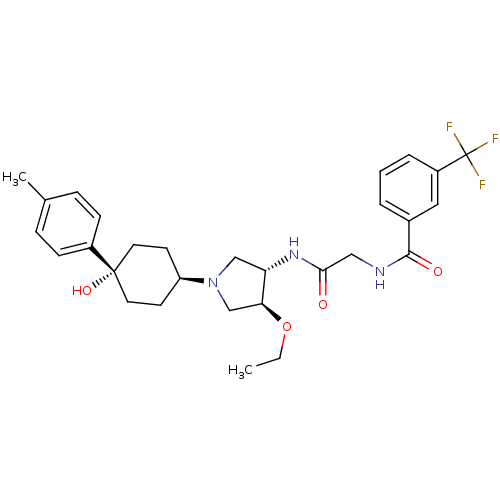
Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Potassium voltage-gated channel subfamily H member 2
Ligand
BDBM50331727
Substrate
n/a
Meas. Tech.
ChEMBL_687881 (CHEMBL1291445)
IC50
11000±n/a nM
Citation
 Xue, CB; Wang, A; Meloni, D; Zhang, K; Kong, L; Feng, H; Glenn, J; Huang, T; Zhang, Y; Cao, G; Anand, R; Zheng, C; Xia, M; Han, Q; Robinson, DJ; Storace, L; Shao, L; Li, M; Brodmerkel, CM; Covington, M; Scherle, P; Diamond, S; Yeleswaram, S; Vaddi, K; Newton, R; Hollis, G; Friedman, S; Metcalf, B Discovery of INCB3344, a potent, selective and orally bioavailable antagonist of human and murine CCR2. Bioorg Med Chem Lett 20:7473-8 (2010) [PubMed] Article
Xue, CB; Wang, A; Meloni, D; Zhang, K; Kong, L; Feng, H; Glenn, J; Huang, T; Zhang, Y; Cao, G; Anand, R; Zheng, C; Xia, M; Han, Q; Robinson, DJ; Storace, L; Shao, L; Li, M; Brodmerkel, CM; Covington, M; Scherle, P; Diamond, S; Yeleswaram, S; Vaddi, K; Newton, R; Hollis, G; Friedman, S; Metcalf, B Discovery of INCB3344, a potent, selective and orally bioavailable antagonist of human and murine CCR2. Bioorg Med Chem Lett 20:7473-8 (2010) [PubMed] Article More Info.:
Target
Name:
Potassium voltage-gated channel subfamily H member 2
Synonyms:
1,3-beta-glucan synthase component GLS2 | Cytochrome P450 3A4 | ERG | ERG1 | Eag-related protein 1 | Ether a-go-go related gene potassium channel (hERG) | Ether-a-go-go-related gene (HERG) | Ether-a-go-go-related gene potassium channel (hERG) | Ether-a-go-go-related gene potassium channel 1 | Ether-a-go-go-related gene potassium channel 1 (HERG) | Ether-a-go-go-related gene potassium channel 1 (hERG1) | Ether-a-go-go-related protein (hERG) | Ether-a-go-go-related protein 1 | Ether-a-go-go-related protein 1 (HERG) | H-ERG | HERG | KCNH2 | KCNH2_HUMAN | Potassium voltage-gated channel subfamily H member 2 (hERG) | Transcriptional regulator ERG | Voltage-gated potassium channel subunit Kv11.1 | eag homolog | hERG Potassium Channel 1 | putative potassium channel subunit
Type:
Multi-pass membrane protein
Mol. Mass.:
126672.65
Organism:
Homo sapiens (Human)
Description:
Q12809
Residue:
1159
Sequence:
MPVRRGHVAPQNTFLDTIIRKFEGQSRKFIIANARVENCAVIYCNDGFCELCGYSRAEVMQRPCTCDFLHGPRTQRRAAAQIAQALLGAEERKVEIAFYRKDGSCFLCLVDVVPVKNEDGAVIMFILNFEVVMEKDMVGSPAHDTNHRGPPTSWLAPGRAKTFRLKLPALLALTARESSVRSGGAGGAGAPGAVVVDVDLTPAAPSSESLALDEVTAMDNHVAGLGPAEERRALVGPGSPPRSAPGQLPSPRAHSLNPDASGSSCSLARTRSRESCASVRRASSADDIEAMRAGVLPPPPRHASTGAMHPLRSGLLNSTSDSDLVRYRTISKIPQITLNFVDLKGDPFLASPTSDREIIAPKIKERTHNVTEKVTQVLSLGADVLPEYKLQAPRIHRWTILHYSPFKAVWDWLILLLVIYTAVFTPYSAAFLLKETEEGPPATECGYACQPLAVVDLIVDIMFIVDILINFRTTYVNANEEVVSHPGRIAVHYFKGWFLIDMVAAIPFDLLIFGSGSEELIGLLKTARLLRLVRVARKLDRYSEYGAAVLFLLMCTFALIAHWLACIWYAIGNMEQPHMDSRIGWLHNLGDQIGKPYNSSGLGGPSIKDKYVTALYFTFSSLTSVGFGNVSPNTNSEKIFSICVMLIGSLMYASIFGNVSAIIQRLYSGTARYHTQMLRVREFIRFHQIPNPLRQRLEEYFQHAWSYTNGIDMNAVLKGFPECLQADICLHLNRSLLQHCKPFRGATKGCLRALAMKFKTTHAPPGDTLVHAGDLLTALYFISRGSIEILRGDVVVAILGKNDIFGEPLNLYARPGKSNGDVRALTYCDLHKIHRDDLLEVLDMYPEFSDHFWSSLEITFNLRDTNMIPGSPGSTELEGGFSRQRKRKLSFRRRTDKDTEQPGEVSALGPGRAGAGPSSRGRPGGPWGESPSSGPSSPESSEDEGPGRSSSPLRLVPFSSPRPPGEPPGGEPLMEDCEKSSDTCNPLSGAFSGVSNIFSFWGDSRGRQYQELPRCPAPTPSLLNIPLSSPGRRPRGDVESRLDALQRQLNRLETRLSADMATVLQLLQRQMTLVPPAYSAVTTPGPGPTSTSPLLPVSPLPTLTLDSLSQVSQFMACEELPPGAPELPQEGPTRRLSLPGQLGALTSQPLHRHGSDPGS
Inhibitor
Name:
BDBM50331727
Synonyms:
CHEMBL1289203 | N-(2-((3S,4S)-4-ethoxy-1-(cis-4-hydroxy-4-p-tolylcyclohexyl)pyrrolidin-3-ylamino)-2-oxoethyl)-3-(trifluoromethyl)benzamide
Type:
Small organic molecule
Emp. Form.:
C29H36F3N3O4
Mol. Mass.:
547.609
SMILES:
CCO[C@H]1CN(C[C@@H]1NC(=O)CNC(=O)c1cccc(c1)C(F)(F)F)[C@H]1CC[C@](O)(CC1)c1ccc(C)cc1 |r,wU:3.2,25.26,wD:7.8,28.30,(4.62,-48.68,;3.37,-47.78,;3.52,-46.25,;2.27,-45.35,;.81,-45.83,;-.1,-44.58,;.81,-43.34,;2.26,-43.81,;3.6,-43.04,;4.93,-43.81,;4.93,-45.35,;6.26,-43.04,;7.6,-43.81,;8.93,-43.04,;8.93,-41.5,;10.26,-43.81,;11.59,-43.04,;12.93,-43.81,;12.93,-45.36,;11.6,-46.13,;10.26,-45.36,;11.6,-47.67,;10.26,-48.44,;12.93,-48.44,;11.58,-49.2,;-1.64,-44.58,;-2.41,-45.92,;-3.95,-45.92,;-4.72,-44.58,;-5.13,-43.09,;-3.94,-43.25,;-2.41,-43.25,;-6.25,-44.58,;-7.02,-43.24,;-8.56,-43.24,;-9.33,-44.57,;-10.87,-44.57,;-8.56,-45.91,;-7.02,-45.91,)|