Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Histamine H3 receptor
Ligand
BDBM50110288
Substrate
n/a
Meas. Tech.
ChEMBL_489537 (CHEMBL986460)
Ki
0.5±n/a nM
Citation
 Roche, O; Nettekoven, M; Vifian, W; Sarmiento, RM Refinement of histamine H3 ligands pharmacophore model leads to a new class of potent and selective naphthalene inverse agonists. Bioorg Med Chem Lett 18:4377-9 (2008) [PubMed] Article
Roche, O; Nettekoven, M; Vifian, W; Sarmiento, RM Refinement of histamine H3 ligands pharmacophore model leads to a new class of potent and selective naphthalene inverse agonists. Bioorg Med Chem Lett 18:4377-9 (2008) [PubMed] Article More Info.:
Target
Name:
Histamine H3 receptor
Synonyms:
G-protein coupled receptor 97 | GPCR97 | HH3R | HISTAMINE H3 | HRH3 | HRH3_HUMAN | Histamine H3 receptor (H3) | Histamine H3L | Histamine receptor (H3 and H4)
Type:
G Protein-Coupled Receptor (GPCR)
Mol. Mass.:
48691.47
Organism:
Homo sapiens (Human)
Description:
Binding assays were using CHO cells stably expressing hH3R receptors.
Residue:
445
Sequence:
MERAPPDGPLNASGALAGEAAAAGGARGFSAAWTAVLAALMALLIVATVLGNALVMLAFVADSSLRTQNNFFLLNLAISDFLVGAFCIPLYVPYVLTGRWTFGRGLCKLWLVVDYLLCTSSAFNIVLISYDRFLSVTRAVSYRAQQGDTRRAVRKMLLVWVLAFLLYGPAILSWEYLSGGSSIPEGHCYAEFFYNWYFLITASTLEFFTPFLSVTFFNLSIYLNIQRRTRLRLDGAREAAGPEPPPEAQPSPPPPPGCWGCWQKGHGEAMPLHRYGVGEAAVGAEAGEATLGGGGGGGSVASPTSSSGSSSRGTERPRSLKRGSKPSASSASLEKRMKMVSQSFTQRFRLSRDRKVAKSLAVIVSIFGLCWAPYTLLMIIRAACHGHCVPDYWYETSFWLLWANSAVNPVLYPLCHHSFRRAFTKLLCPQKLKIQPHSSLEHCWK
Inhibitor
Name:
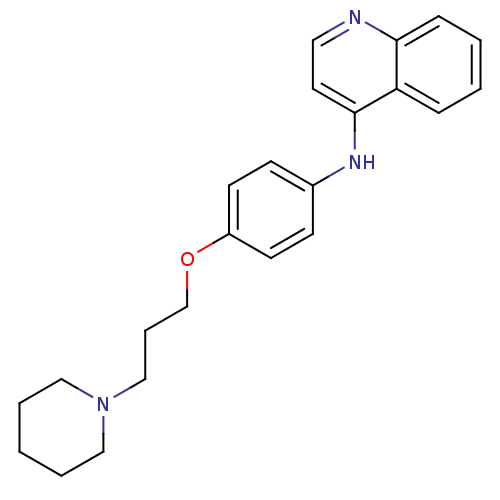
BDBM50110288
Synonyms:
CHEMBL15153 | N-(4-(3-(piperidin-1-yl)propoxy)phenyl)quinolin-4-amine | [4-(3-Piperidin-1-yl-propoxy)-phenyl]-quinolin-4-yl-amine; Oxalic acid
Type:
Small organic molecule
Emp. Form.:
C23H27N3O
Mol. Mass.:
361.48
SMILES:
C(COc1ccc(Nc2ccnc3ccccc23)cc1)CN1CCCCC1