Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Hemagglutinin/Neuraminidase
Ligand
BDBM493570
Substrate
n/a
Meas. Tech.
Cytopathic Effect Assay (CPE Assay)
EC50
1.22±n/a nM
Citation
More Info.:
Target
Name:
Hemagglutinin/Neuraminidase
Synonyms:
H1N1
Type:
Protein
Mol. Mass.:
n/a
Description:
n/a
Components:
This complex has 2 components.
Component 1
Name:
Neuraminidase
Synonyms:
n/a
Type:
Protein
Mol. Mass.:
51699.94
Organism:
n/a
Description:
Q20N24
Residue:
469
Sequence:
MNPNQKIITIGSICMVVGIISLILQIGNIISIWISHSIQTGSQNHTGICNQSIITYKNSTWVNQTYVNISNTNVVAGKGTTSVILAGNSSLCPIRGWAIYSKDNGIRIGSKGDVFVIREPFISCSHLECRTFFLTQGALLNDKHSNGTVKDRSPYRALMSCPVGEAPSPYNSRFESVAWSACACHDGMGWLTIGISGPDDEAVAVLKYNGIITETIKSWRKKILRTQESECVCVNGSCFTIMTDGPSDGPASYKIFKIEKGKVTKSIELDAPNSHYEECSCYPDTGKVMCVCRDNWHGSNRPWVSFDQNLDYQIGYICSGVFGDNPRPKDGKGSCGPVYVDGANGVKGFSYRYGNGVWIGRTKSDSSRQGFEMIWDPNGWTETDSNFFVKQDIVAMTDWSGYSGSFVQHPELTGLDCMRPCFWVELIRGRPKEKTIWTSGSSISFCGVNSDTVDWSWPDGAELPFTIDK
Component 2
Name:
Hemagglutinin
Synonyms:
n/a
Type:
Protein
Mol. Mass.:
63515.65
Organism:
n/a
Description:
Q20N27
Residue:
566
Sequence:
MKARLLVLLCALAATDADTICIGYHANNSTDTVDTILEKNVTVTHSVNLLEDSHNGKLCRLKGIAPLQLGKCNIAGWILGNPECESLLSERSWSYIVEIPNSENGTCYPGDFTDYEELREQLSSVSSFERFEIFPKESSWPKHNTARGVTAACSHAGKSSFYRNLLWLTEKDGSYPNLKNSYVNKKGKEVLVLWGVHHPSSIKEQQTLYQKENAYVSVVSSNYNRRFTPEIAERPKVRDQAGRMNYYWTLLEPGDTIIFEANGNLIAPWYAFALSRGFGSGIITSNASMHECDTKCQTPQGAINSSLPFQNIHPVTIGECPKYVRSTKLRMVTGLRNIPSIQSRGLFGAIAGFIEGGWTGMIDGWYGYHHQNEQGSGYAADQKSTQNAINGITNKVNSVIEKMNTQFTAVGKEFNNLEKRMENLNKKVDDGFLDIWTYNAELLILLENERTLDFHDSNVKNLYEKVKSQLRNNAKEIGNGCFEFYHKCNNECMESVKNGTYDYPKYSEESKLNREKIDGVKLESMGVYQILAIYSTVASSLVLLVSLGAISFWMCSNGSLQCRICI
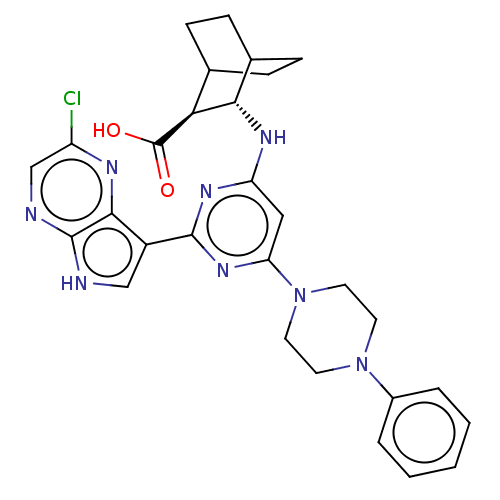
Inhibitor
Name:
BDBM493570
Synonyms:
US10987354, Example 39
Type:
Small organic molecule
Emp. Form.:
C29H31ClN8O2
Mol. Mass.:
559.062
SMILES:
OC(=O)[C@H]1C2CCC(CC2)[C@@H]1Nc1cc(nc(n1)-c1c[nH]c2ncc(Cl)nc12)N1CCN(CC1)c1ccccc1 |r,wU:3.2,wD:10.12,(5.27,1.76,;6.35,.66,;7.84,1.04,;5.93,-.82,;6.93,-2.11,;6.53,-3.6,;5.05,-4,;3.96,-2.91,;5.05,-1.82,;5.82,-3.15,;4.36,-1.42,;3.27,-.33,;1.78,-.73,;.69,.36,;-.8,-.04,;-1.2,-1.53,;-.11,-2.62,;1.38,-2.22,;-.51,-4.11,;.4,-5.35,;-.51,-6.6,;-1.97,-6.12,;-3.3,-6.89,;-4.64,-6.12,;-4.64,-4.58,;-5.97,-3.81,;-3.3,-3.81,;-1.97,-4.58,;-1.89,1.05,;-1.49,2.54,;-2.58,3.62,;-4.06,3.23,;-4.46,1.74,;-3.37,.65,;-5.15,4.31,;-4.75,5.8,;-5.84,6.89,;-7.33,6.49,;-7.73,5,;-6.64,3.92,)|