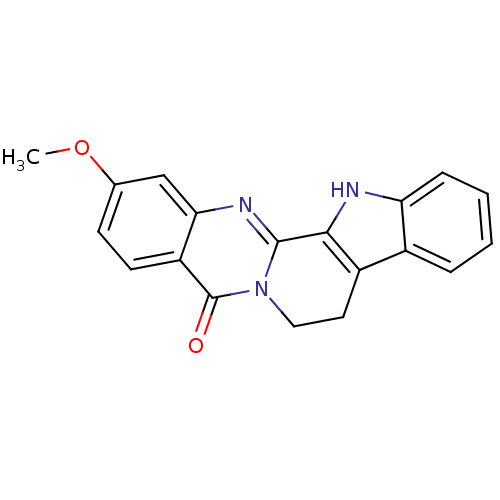
BDBM50131043 2-Methoxy-8,13-dihydro-7H-indolo[2',3':3,4]pyrido[2,1-b]quinazolin-5-one::2-methoxyruteacaprine::CHEMBL312248
SMILES COc1ccc2c(c1)nc1-c3[nH]c4ccccc4c3CCn1c2=O
InChI Key InChIKey=JDYVBLKLNUFKBD-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50131043
Found 3 hits for monomerid = 50131043
TargetCytochrome P450 1A1(Homo sapiens (Human))
National Research Institute Of Chinese Medicine
Curated by ChEMBL
National Research Institute Of Chinese Medicine
Curated by ChEMBL
Affinity DataIC50: 850nMAssay Description:Inhibition of Cytochrome P450 1A1 enzyme in bacterial membrane expressing human P450sMore data for this Ligand-Target Pair
TargetCytochrome P450 1B1(Homo sapiens (Human))
National Research Institute Of Chinese Medicine
Curated by ChEMBL
National Research Institute Of Chinese Medicine
Curated by ChEMBL
Affinity DataIC50: 170nMAssay Description:Inhibition of Cytochrome P450 1B1 enzyme in bacterial membrane expressing human P450sMore data for this Ligand-Target Pair
TargetCytochrome P450 1A2(Homo sapiens (Human))
National Research Institute Of Chinese Medicine
Curated by ChEMBL
National Research Institute Of Chinese Medicine
Curated by ChEMBL
Affinity DataIC50: 76nMAssay Description:Inhibition of Cytochrome P450 1A2 enzyme in bacterial membrane expressing human P450sMore data for this Ligand-Target Pair